Promising advances in fetal therapy for vein of Galen malformation

In 2024, Megan Ingram* of California and her husband were preparing for the birth of their third child when a 34-week ultrasound revealed a potential complication. Their obstetrics team suspected a vein of Galen malformation (VOGM) — a rare vascular condition involving significantly elevated blood flow to the head because of direct connections between arteries and veins in the developing brain. If left untreated until after delivery, VOGM commonly leads to congestive heart failure, pulmonary hypertension, and brain injury.
A first and a turning point
In 2023, Dr. Orbach and the team at Boston Children’s Fetal Care and Surgery Center performed the first fetal embolization for vein of Galen malformation (VOGM) in the U.S. Baby Denver was born one week later. Her safe arrival marked a watershed moment in the treatment of this high-risk condition.
Read her story.
Amid a flurry of Googling, consultations, and what felt like conflicting opinions, one specialist mentioned a clinical trial underway at Boston Children’s Hospital studying fetal embolization, a minimally invasive prenatal procedure aimed at reducing the abnormal blood flow in the brain caused by VOGM and lowering the risk of heart and brain damage. The Ingrams reached out to Dr. Darren Orbach, the trial’s lead investigator and co-director of Boston Children’s Cerebrovascular Surgery and Interventions Center. After confirming they met the study criteria, they soon became some of the first participants in the early-stage clinical trial.
“Dr. Orbach and his team knew how to address VOGM and how to speak about it,” Megan says. “I can’t overstate the relief that comes with not having to be a steward [to our care providers] for this very rare thing.”
At 37 weeks, Megan underwent successful fetal embolization at Boston Children’s. The procedure went as planned, allowing her to continue the pregnancy until 38 weeks, when she delivered her son by scheduled C-section. After delivery, her son stayed in Boston Children’s NICU for five weeks and underwent additional embolization to manage the malformation.
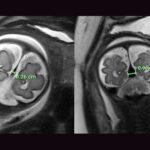
Today, he’s a happy and active one-year-old — eating and moving well, communicating, and meeting milestones. Follow-up MRIs over the past year have shown no signs of brain injury and virtually no flow left in the VOGM.
“It has been one of the most profound experiences of my life, getting to watch my son get better,” Megan says.
Early trial results published in JAMA
In September 2025, the Boston Children’s team published outcomes from the first seven trial patients in JAMA, including the Ingrams’ son.
Key findings include:
- Successful fetal embolization in five of the seven patients
- A decrease in neonatal mortality from an expected 90 percent to 43 percent
- No neurodevelopmental delays observed in the surviving embolized patients to date
These early results point to the potential of fetal embolization as a viable option for managing VOGM prenatally.
“Our data suggest that this approach is certainly feasible, in other words, that the procedure may be both safe and effective,” says Orbach. “In this first group of patients, we’ve seen significant improvement in the outcomes for affected fetuses, both in terms of survival and, equally important, in terms of great neurological outcomes. However, we need to complete a safety and efficacy trial to be confident that fetal embolization is something we should recommend.”
Rethinking when to intervene
Fetal embolization for VOGM remains an emerging therapy. However, trial results suggest a potential shift in how providers manage prenatally diagnosed VOGMs — moving from treating complications after birth to thinking about earlier intervention aimed at preventing damage before it occurs. The Boston Children’s team is continuing to evaluate long-term neurological outcomes and weigh the benefits of early intervention against the risks of preterm delivery and other complications.
“Our goal is early, effective treatment that makes this high-risk diagnosis routinely survivable,” said Orbach.
What it means to say “yes” to a new approach
For the Ingrams, the decision to join the clinical trial wasn’t easy. They had questions and initial doubts about pursuing a procedure that was still being studied.
“It’s a difficult feeling,” Megan says. “Choosing something experimental but that also feels like the safest choice.”
They faced the added strain of leaving their young children, stepping away from work, and being far from home. Moving forward with the trial came from a need to act. They thought back to the day of their son’s diagnosis — a moment so overwhelming that even their doctors cleared their schedules to focus entirely on what to do next, despite having no clear answers. In the days that followed, uncertainty remained, with differing opinions on how to proceed. The trial wasn’t a guarantee, but it offered an active direction — and the chance to pursue every option.
“I remember thinking, ‘we’ve come this far, and there’s this guy with a trial in Boston’,” Megan recalls. “I said, ‘Let’s just try.’ I think about that decision every day.”
*Names have been changed
Learn more about the fetal embolization for VOGM and the Fetal Care and Surgery Center.
Related Posts :
-

Changing the world: Baby Denver leads the way after first-of-its-kind procedure for VOGM
Denver Coleman is less than 2 months old, but she’s already helped blaze a trail for other children and families, ...
-

Years in the making: Team performs successful fetal intervention for VOGM
On an ordinary Wednesday in March, a team of specialists from two institutions made the extraordinary happen: a first-of-its-kind intervention ...
-

'To do what’s best for Marley': One family’s experience with a vein of Galen malformation
Last summer, Savannah and Brian were eagerly awaiting the birth of their first child. Savannah was scheduled to deliver their ...
-

Could the falcine sinus hold the key to vein of Galen outcomes?
A Boston Children’s Hospital study uncovers how fetal magnetic resonance imaging (MRI) could be a game-changer in predicting outcomes ...