Capturing SARS-CoV-2’s shape-shifting spike protein
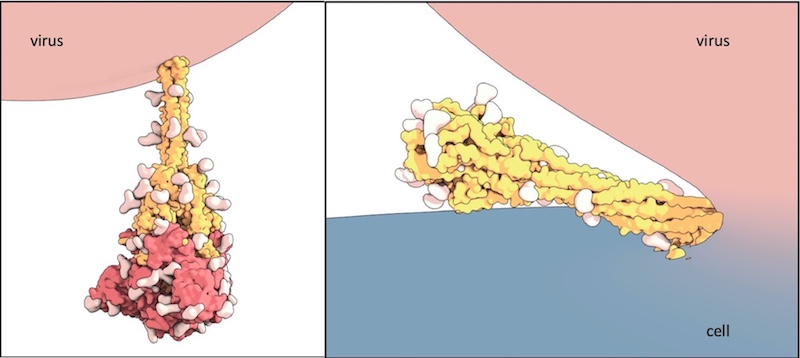
The rod-like spike proteins on the surface of SARS CoV-2 are the tip of the spear of the COVID-19 pandemic. The spikes bind to human cells via the ACE2 receptor and then dramatically change shape. They jack-knife, folding in on themselves to fuse their own membrane with the membrane of our cells. And that opens the door to coronavirus infection.
· Cryogenic electron microscopy freeze-frames SARS-CoV-2, the coronavirus behind COVID-19, before and after it changes shape.
· The second shape may help shield the virus from the environment and the immune system.
· Knowing both shapes will aid in vaccine development.
The spike protein is the main protein targeted by our antibodies and the protein used in most vaccines now in clinical trials. A study led by Boston Children’s Hospital freeze-frames the spike protein for the first time in its “before” and “after” shapes. The two forms are published today in Science.
In the process, the study captured some surprise features in the “after” shape that may have implications for vaccine and therapeutic development. The investigators, led by Bing Chen, PhD, believe these features may help SARS-CoV-2 hide from the immune system and survive longer in the environment.
Freeze-framing SARS-CoV-2 spikes
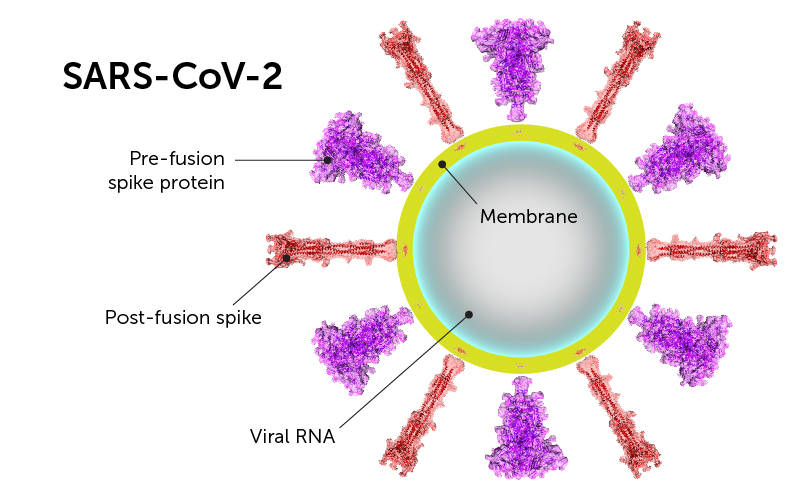
Using the technique of cryogenic electron microscopy, Chen and colleagues established the molecular structure of the spike protein, both before and after fusion of the virus and cell membranes. In the “after,” post-fusion state, the protein assumes a rigid hairpin shape, they showed.
Related: Sturdier spikes may explain SARS-CoV-2 variants’ faster spread: A mutation carried by the UK, South Africa, and Brazil SARS-CoV-2 variants stabilizes the spike protein, rendering the virus better able to infect us.
Intriguingly, they also found that the spike can go from its original “before” shape into the “after” form prematurely, without the virus binding to a cell at all.
“We propose that there are two routes for the conformational changes,” says Chen, in Boston Children’s Division of Molecular Medicine. “One is ACE2 dependent, and allows the virus to enter a host cell. The second is ACE2 independent.”
A coronavirus defense mechanism?
As a result of the spontaneous shape change, coronavirus particles often bear both forms of the spike protein. The rigid “after” form protrudes slightly more from the virus surface. Chen suggests that being able to assume this alternate shape may help keep SARS-CoV-2 from breaking down, when it lands on a surface for example. That could explain why the virus appears to remain viable on various surfaces for hours to days.
“Most viruses don’t survive long outside the host,” Chen says. “We think the rigid structure of these post-fusion spikes protects the virus.”
Evading immune detection
The researchers speculate that having some spikes take the post-fusion form prematurely may also protect SARS-CoV-2 from our immune system, inducing antibodies that are non-neutralizing and ineffective in containing the virus. In effect, the post-fusion spikes may act as decoys that distract the immune system.
The team was also surprised to find that the post-fusion spikes, similar to the pre-fusion spikes, have glycans, or sugar molecules, at evenly spaced locations on their surface. Glycans are another feature that helps the virus avoid immune detection.
Chen believes his team’s findings have implications for vaccine development. Current vaccines that use the spike protein to stimulate the immune system may have varying mixes of the pre- and post-fusion forms, he says. And this may limit their protective efficacy.
“We need to think about how to stabilize the spike protein,” he says. “If the protein is not stable, you may be able to induce antibodies, but they will be less effective in terms of blocking the virus. There may be batch-to-batch variation.”
Below, an artists’ rendering of how the shape change of the spike protein drives the membrane fusion process, based on data from Chen’s team and other sources:
Lessons from HIV
Chen’s many years of research on HIV have helped give his team a leg up on studying SARS-CoV-2. Both viruses are what’s known as envelope viruses, and need to fuse their membranes with those of the cells they’re infecting. Both use the same jack-knifing shape change, and both have spike proteins on their surface that are decorated with sugars. Finally, HIV vaccine developers struggle with the challenge of developing neutralizing antibodies. HIV, too, distracts the immune system into creating multiple antibodies that don’t shut down the virus.
“I think SARS-CoV-2 is probably an easier target than HIV, but we will have to see,” says Chen. “If this first round of vaccines does not work well in phase 3 trials, this new understanding of the spike structure may help us design stronger vaccines.”
Yongfei Cai and Jun Zhang in Chen’s lab were first authors on the paper. Researchers from the Harvard Cryo-EM Center for Structural Biology and the Blavatnik Institute of Harvard Medical and SBGrid Consortium also collaborated on the study. The work was supported by the National Institutes of Health and a COVID19 Award from the Massachusetts Consortium on Pathogen Readiness.
Learn more about Boston Children’s response to COVID-19 and coronavirus research at the hospital.
Related Posts :
-

Creating the next generation of mRNA vaccines
During the COVID-19 pandemic, mRNA vaccines came to the rescue, developed in record time and saving lives worldwide. Researchers in ...
-

Tracking influenza in its first battleground: The nose
The answer to curbing influenza could be right under our noses — or, more accurately, inside them. New research maps happenings ...
-

A deeper understanding of inflammatory pain could reveal new solutions
Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen are the main go-to for inflammatory pain caused by wounds, infection, sunburn, arthritis, ...
-

Reversing the trend: Easing the mental health boarding crisis in emergency rooms
Anxiety, depression, and suicide attempts have been rising over the past decade, especially among teens, often landing them in emergency ...