A heart valve that grows along with a child could reduce invasive surgeries

Clinical trials have started for the first prosthetic pulmonary valve replacement that is specifically designed for pediatric patients and can expand over time inside a child’s anatomy.
Instead of having invasive replacement surgeries every few years, as is the practice now, a child can have the valve fitted to their individual body size and, if needed, adjusted for size through a minimally-invasive transcatheter balloon dilation procedure to help maintain blood flow. They potentially wouldn’t need another replacement procedure until adulthood.
The device, known as the Autus Valve, was invented at Boston Children’s. It was first implanted in a young patient at Nationwide Children’s Hospital in late 2021 as part of a collaborative clinical study involving Boston Children’s, Nationwide Children’s, and New York-Presbyterian/Columbia University Irving Medical Center. “It’s exciting and incredibly motivating that we’re at the stage where we can actually see the device helping patients,” says the device’s inventor, Sophie-Charlotte Hofferberth, MD.
Boston Children’s cardiac catheterization team has already made other significant advancements in transcatheter valve replacement technology, and the Autus Valve continues that pace of innovation, according to Christopher Baird, MD, director of the hospital’s Congenital Heart Valve Program.
Mimicking the design of human venous valves
An estimated 110,000 children in the U.S. have congenital pulmonary valve disease, which impedes the flow of blood between the heart and lungs. Pulmonary valves that are narrowed or leaking and can’t be treated effectively with a catheter are usually replaced with a prosthetic valve. But existing prosthetic pulmonary valves are adult-sized and fixed in diameter, usually requiring a child to have these devices replaced as they grow.

Hofferberth was tasked with creating a valve that could accommodate the growth of a child when she joined the research laboratory of Pedro J. del Nido, MD, chief of Boston Children’s Cardiac Surgery Department, in 2016.
Commercially available prosthetic heart valves have three leaflets that serve as flaps to control blood flow, mirroring the tri-leaflet structure of the human aortic valve. A device that mimicked the bi-leaflet function of a venous valve in leg veins showed promise to Hofferberth and colleagues. That’s because a venous valve’s two elastic flaps have the ideal geometry to maintain proper closure and one-way blood flow even when veins in the leg expand in diameter. Believing this process could also work in the heart, Hofferberth studied the geometric profile of the human venous valve and used this as the basis for creating a valve prototype.
Designing a size-adjustable valve that will last
Hofferberth and her team collaborated with Elazer Edelman, MD, PhD, of the Massachusetts Institute of Technology. They implanted prototypes of the replacement pulmonary valve in growing lambs, and those studies showed the device can be fitted and then expanded in sync with the growth of heart anatomy. The valve also maintained the control of blood flow — without stretching and compromising the device’s frame or material. The device’s two leaflets are made of a polymer with a long track record of use as a pediatric pulmonary valve leaflet. “That gave us a lot of confidence, using a well-known synthetic material that has proven long-term results in children,” she says.
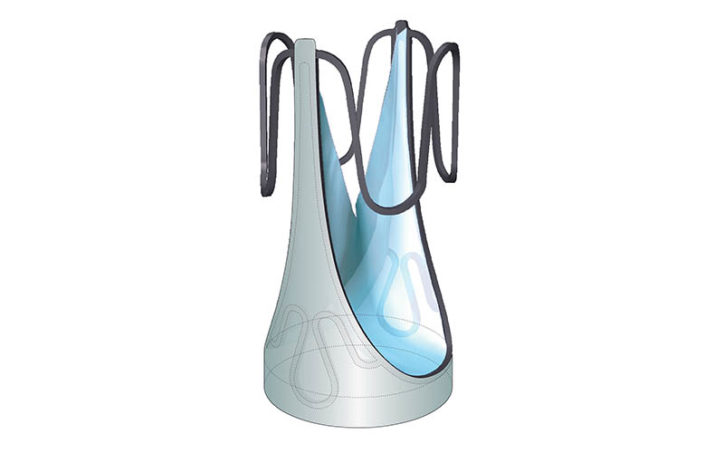
Doctors can adjust the valve diameter to match a patient’s heart anatomy before it is implanted. Once the device is implanted, cardiac catheterization specialists can use a catheter balloon to expand the valve if it becomes too small after a child’s growth spurt. The doctors use an echocardiogram to assess the valve’s integrity and how well it is controlling the flow of blood.
“If a valve expansion is needed after the device is implanted, we anticipate a child would recover from the procedure within a few hours,” says Hofferberth, who is no longer with Boston Children’s.
Aiming for children’s long-term heart health
Boston Children’s is now holding FDA-approved early clinical studies on the valve’s effectiveness in children from the age of 18 months to 16 years, along with Nationwide Children’s and New-York Presbyterian/Columbia University Irving Medical Center. Since the initial patient, several other children received Autus Valve implants; Boston Children’s had its first implantation procedure in late December.
Learn more about the clinical trial
If you have a child who might be eligible to participate in the Autus Valve clinical trial and would like more information, please contact Dr. Christopher Baird, director of the Benderson Family Heart Center Congenital Heart Valve Program and director of the trial at Boston Children’s. He can be reached at chris.baird@cardio.chboston.org
Hofferberth intends to follow the initial study with a larger clinical trial, with the goal of seeking FDA approval to make the device commercially available.
“There is a huge need for better solutions for children with valve disease,” Hofferberth says. “A pulmonary valve that can be adjusted for size could give young patients a bridge through childhood and have a huge impact on their long-term quality of life.”
Dr. del Nido is a co-founder and board member of Autus Valve Technologies, Inc., and has equity in the company. Boston Children’s Hospital is also an equity holder in Autus Valve Technologies, Inc. As in all research studies, the hospital has taken, and will continue to take, all necessary steps to ensure research subject safety and the validity and integrity of the information obtained by this research.
Learn more about the Department of Cardiac Surgery or refer a patient to the Benderson Family Heart Center.
Related Posts :
-

A new lens on cardiac surgery could help prevent arrhythmia
Sometimes, a change in perspective can lead to a medical breakthrough. A type of microscopy typically used to detect cancer ...
-

"Seeing" the unseen: A way to pinpoint elusive cardiac conduction tissue
When patients with congenital heart issues have an operation, surgeons have to proceed with an “eye of faith” as they ...
-

From South Africa to Boston: Kyleigh's four-year search for good heart health
Kyleigh Kista had three open-heart surgeries in just the first 18 months of her life. But instead of progressing, her ...
-

Along the way to heart surgery, Liam found a name for his new teddy bear
After a complicated delivery in April 2021, Chelsea Allis had recovered and was finally able to bond with her infant son, ...