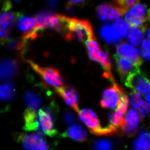
Mitochondrial transfer restores heart muscle — but how?
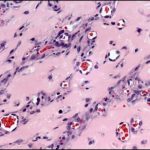
Transferring mitochondria from a patient’s healthy skeletal muscle to damaged, ischemic heart tissue has been shown to restore heart muscle, increase energy production, and improve ventricular function. After pioneering preclinical work by James McCully, PhD, at Boston Children’s Hospital about a decade ago, cardiac surgeons led by Sitaram Emani, MD, have been testing it as a way to help wean children with congenital heart disease and ischemia-reperfusion injury off ECMO.
“We realized the probability of recovery was much higher if we added mitochondria,” Emani says. To date, 16 children have undergone autologous mitochondria transplantation. Of these, 80 percent were able to come off ECMO, compared with a historical rate of 40 percent.
But mitochondrial transfer has faced skepticism — in part because no one really knew why it works.
“We assumed it was mitochondria going into cells and taking over and generating all of the cell’s power,” says Emani. “But what didn’t make sense was that we only needed very small amounts of mitochondria for the heart muscle to recover. The math didn’t add up.”
A study led by Juan Melero-Martin, PhD, a researcher in the Department of Cardiac Surgery, found a surprising explanation, reported in Nature on May 1. The transferred mitochondria trigger the cell to destroy its low-performing mitochondria through autophagy — a kind of cellular housekeeping. This gives cells a better pool of mitochondria, improving their bioenergetics and fitness. This insight could ultimately improve care for broad range of heart conditions.
Tracking what mitochondria are doing
The Melero-Martin lab began with a different, long-standing goal in mind: growing networks of blood vessels to supply engineered tissues and make tissue transplants more effective. Such blood vessel networks could also deliver drugs or gene therapies or provide a home for transplanted cells, like insulin-producing cells for diabetic patients.
To build the networks, the lab worked with endothelial cells, which make up blood vessels. But an extra boost was needed. “We realized that endothelial cells need supporting cells such as mesenchymal cells to help them engraft into tissues in the body,” Melero-Martin says.
The team then showed that engraftment of endothelial cells depended on the transfer of mitochondria from the accompanying mesenchymal cells. When they isolated the mitochondria and incubated them directly with the endothelial cells, the cells became more fit and better able to engraft. “But none of our experiments got to the core of what happened to the cells and how they achieved benefits from the mitochondria,” Melero-Martin says.
So the researchers decided to tag the mitochondria and track them after putting them into cells. “A few days later, there was no trace of them; they were getting degraded,” Melero-Martin says. “Yet we still saw an increase in energy in the cells. We were puzzled.”
The latest work, just reported in Nature, showed that the influx of mitochondria flips a switch that makes the cells “clean house,” digesting old mitochondria and making a fresh supply. But for clinical applications, mitochondrial transfer seems like a lot of trouble to rejuvenate cells. Could autophagy be triggered without needing to actually transplant mitochondria?
Melero-Martin’s lab is investigating different possibilities. They include engineering off-the-shelf mitochondria usable in any patient, using just a portion of the mitochondria, or directly triggering molecular pathways involved in autophagy. (The study identified one such pathway, PINK1-Parkin.)
Applying mitochondria to heart transplant
Meanwhile, Emani is now investigating whether mitochondrial transfer could improve the success of cardiac transplantation when the heart is donated after circulatory death (DCD). DCD hearts could potentially expand the donor pool, but have ischemic damage and thus are difficult to transplant.
“We know that patients have some period of ischemia-reperfusion injury after transplantation,” Emani says. “We think treatment with mitochondria will help with their recovery.”
McCully and Emani previously showed that mitochondrial transfer preserves myocardial function in an animal model of DCD transplantation. Now, through a partnership with New England Donor Services, they are working with human DCD hearts not being used for transplantation. This preclinical protocol entails reanimating the heart, delivering either mitochondria or placebo, and measuring heart function.
“If it works, it would really expand the ability for a donation to be utilized,” Emani says. “The next step would be a clinical trial.”
Emani also has his eye on using mitochondria for hypoplastic left heart syndrome (HLHS). He and his colleagues just completed a small randomized trial looking at transfer of mesenchymal progenitor cells, pretreated with mitochondria, into the left ventricular endocardium to help patients with HLHS achieve biventricular circulation. He thinks the mitochondria could improve engraftment of the mesenchymal cells, which he hopes in turn will potentiate ventricular function and stimulate heart muscle remodeling.
“We’re always thinking about cell-based therapies for heart disease and optimizing grafts, like new leaflets or patches for the heart, by providing a vascular supply,” Emani says. “Juan’s results provide a missing link in our understanding of how the transplanted mitochondria are working.”
Learn more about the Department of Cardiac Surgery or refer a patient.
Related Posts :
-

Long-term hemophilia treatment could lie in patients' own cells
Children (and adults) with hemophilia are slow to form blood clots, so are at constant risk for uncontrolled bleeding. Even ...
-

Avoiding the needle: Engineering blood vessels to secrete drugs
People who rely on protein-based drugs often have to endure IV hookups or frequent injections, sometimes several times a week. ...
-

A 'super' new heart surgery for a super kid
When you’re the first person in the world to undergo a new type of heart surgery, you don’t ...
-

Mending injured hearts: Lessons from newborns?
When the heart is injured, as in a myocardial infarction, the damaged heart muscle cannot regenerate — instead, scar tissue forms. ...