Could we intervene in Huntington’s disease before symptoms appear?
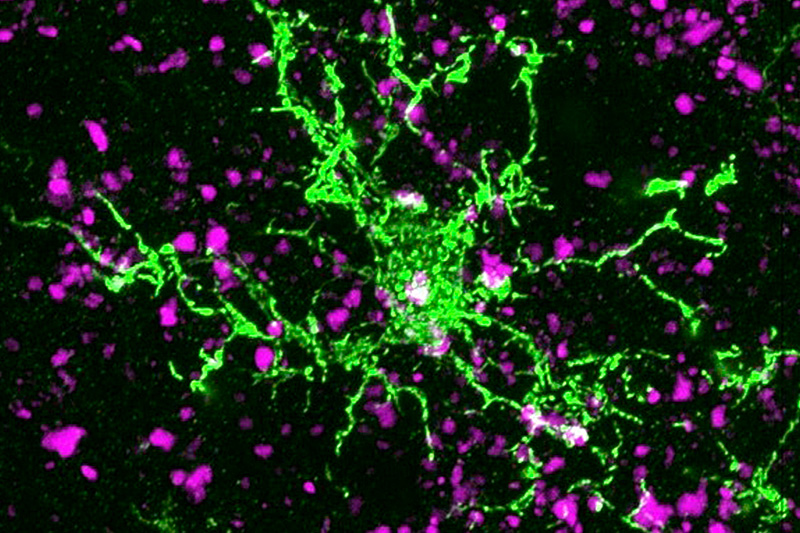
Huntington’s disease is the most common single-gene neurodegenerative disorder and is characterized by motor and cognitive deficits and psychiatric symptoms. Work led by Beth Stevens, PhD, and Dan Wilton, PhD, in the Department of Neurology at Boston Children’s Hospital, now shows that the disease process begins well before symptoms appear.
That raises the possibility of targeting Huntington’s early in people who carry the mutation. The work, just published in Nature Medicine, could also shed light on other neurodegenerative disorders.
Tagging and eliminating synapses
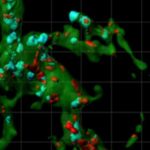
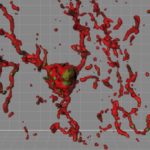
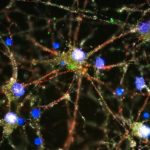
In 2012, the Stevens lab was among the first to show that immune cells in the brain known as microglia engulf and prune synapses during normal brain development, fine-tuning the brain’s connections. The lab also showed that complement proteins, also part of the immune system, tag synapses meant for elimination. Stevens and her team speculated that in diseases involving synapse loss, like Alzheimer’s disease and schizophrenia, this pruning process reactivates abnormally.
However, these conditions are difficult to study: They’re caused by multiple genes and lack good animal models. Huntington’s disease offered an ideal research opportunity.
“Huntington’s has one causal gene, huntingtin, and a very selective and stereotyped pathology allowing us to observe the disease process at very early stages,” says Stevens, a member of the F.M. Kirby Neurobiology Center at Boston Children’s. (She is also affiliated with the Broad Institute’s Stanley Center for Psychiatric Research and the Howard Hughes Medical Institute.) “We were able to ask, is the complement- and microglia-mediated pruning mechanism activated early? And if so, can we intervene?”
Corticostriatal circuit targeted for synapse loss
Using a mouse model and postmortem brain samples from patients with Huntington’s disease, Wilton and colleagues showed that complement proteins and microglia are activated very early in the illness — before cognitive and motor symptoms emerge — and that they target a specific vulnerable brain circuit.
While the mutant huntingtin gene is expressed in every cell, the postmortem brain tissue showed a selective loss of corticostriatal synapses in the basal ganglia. Corticostriatal circuits are known to be involved in movement and in learning what actions lead to positive outcomes or “reward.” The researchers saw increased levels of complement proteins around the synapses in these circuits.
When the team blocked the complement protein C1q in their mouse model — either with an antibody or by genetically deleting the complement receptor CR3 on microglia — they prevented synapse loss. They also prevented cognitive defects in the mice, specifically around visual discrimination learning and cognitive flexibility.
“Some cognitive deficits tend to develop much earlier than motor defects in Huntington’s disease,” notes Wilton. “There is evidence that this occurs in humans, too. Our mouse model does develop some slight motor defects that are also resolved with complement-blocking strategies.”
An early Huntington’s disease biomarker?
The study validates a treatment already in clinical trials for Huntington’s blocking C1q with an antibody. “Dan, for the first time, showed a specific mechanism for corticostriatal synaptic elimination, demonstrating the selective vulnerability of this synaptic connection, and providing insight into what is happening at the earliest stages of the disease,” Stevens says.
Another clinically promising finding: levels of complement proteins were elevated in the cerebrospinal fluid (CSF) of patients with Huntington’s disease even before they developed motor symptoms.
“We’re excited by the idea that we could identify neuroimmune biomarkers that could stratify people at the earliest stage and prioritize some for treatment,” Stevens says. “If you had clinical samples such as CSF, measuring these biomarkers could bring insight into what is happening in the brain.”
Stevens also thinks similar mechanisms and biomarkers may apply to other neurodegenerative disorders such as Alzheimer’s and frontotemporal dementia, which her lab is exploring.
But most immediately, she and Wilton hope to unravel how the huntingtin mutation leads to complement activation to begin with. They know that expression of the mutant gene must be expressed specifically in cortical and striatal neurons to initiate the synaptic elimination mechanism. But how the corticostriatal inputs are selectively targeted remain to be determined.
“Huntington’s is a really nice model to tease this out,” says Stevens. “That’s a major future direction for our group.”
Learn more about Beth Stevens’s research and the F.M. Kirby Neurobiology Center.
Related Posts :
-

Beth Stevens: A transformative thinker in neuroscience
When 2015 MacArthur “genius” grant winner Beth Stevens, PhD, began studying the role of glia in the brain in the 1990s, ...
-

Synapse ‘protection’ signal found; helps to refine brain circuits
The developing brain is constantly forming new connections, or synapses, between nerve cells. Many connections are eventually lost, while others ...
-

Targeting synapse loss in Alzheimer’s to preserve cognition — before plaques appear
Currently, there are five FDA-approved drugs for Alzheimer’s disease, but these only boost cognition temporarily and don’t ...
-

Genetic analysis backs a neuroimmune view of schizophrenia: Complement gone amok
A deep genetic analysis, involving nearly 65,000 people, finds a surprising risk factor for schizophrenia: variation in an immune molecule best ...