Targeting synapse loss in Alzheimer’s to preserve cognition — before plaques appear
Currently, there are five FDA-approved drugs for Alzheimer’s disease, but these only boost cognition temporarily and don’t address the root causes of Alzheimer’s dementia. Many newer drugs in the pipeline seek to eliminate amyloid plaque deposits or reduce inflammation in the brain, but by the time this pathology is detectable, it’s unlikely medications can do much to slow the disease.
New research published in Science today suggests several ways that Alzheimer’s could be targeted much earlier to preserve cognitive function — before plaques or inflammation are evident.
Working with mouse models of Alzheimer’s, Beth Stevens, PhD and Soyon Hong, PhD of Boston Children’s Hospital’s F.M. Kirby Neurobiology Center and their colleagues lay out how brain connections (synapses) begin to unravel in the earliest stages of the disease.
“We know synapse loss precedes a lot of the pathology in Alzheimer’s and is a strong correlate of cognitive decline,” says Stevens, also an Assistant Professor at Harvard Medical School and Boston Children’s Department of Neurology. “A big question has been how synapses are lost.”
The young brain informing the old
Stevens, Hong and colleagues looked at Alzheimer’s through an unusual lens: normal brain development in infancy and childhood. Through more than a decade of research, which helped earn her a 2015 MacArthur “genius” award, Stevens has shown that normal developing brains have a built-in system to “prune” synapses that aren’t needed as they build their circuitry.
Stevens and Hong now show in multiple mouse models of Alzheimer’s that similar pruning mechanisms are wrongly activated later in life. By blocking these mechanisms, they were able to reduce synapse loss in the mice before amyloid plaque deposits could be observed.
“Understanding a normal developmental process deeply has provided us with novel insight into how to protect synapses in Alzheimer’s and potentially a host of other diseases,” Stevens says.
The work may have relevance for other conditions involving synapse loss, such as frontotemporal dementia, Huntington’s disease, schizophrenia and glaucoma, she adds.
Neuro-immune synapse destruction
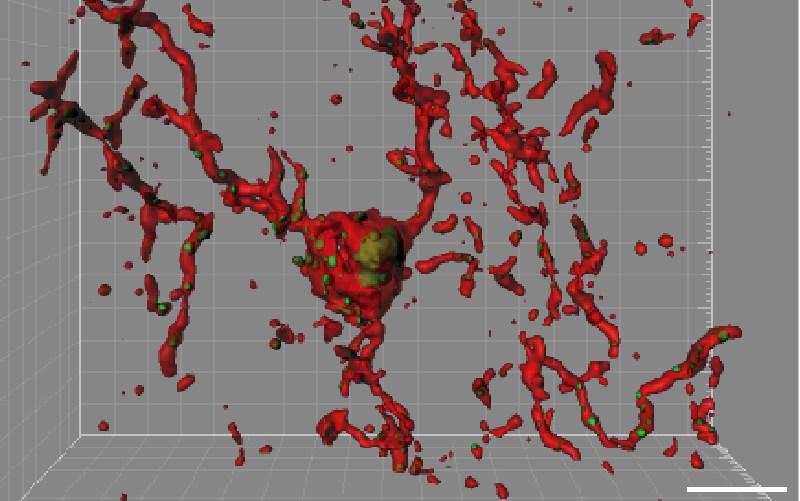
In the mouse models of Alzheimer’s, the team found that synapse loss requires activation of the protein C1q, which “tags” synapses for elimination. C1q became visibly more abundant around vulnerable synapses in the mice. Cells known as microglia then came in and “ate” the tagged synapses, as they do during normal brain development.

When Stevens and colleagues blocked C1q, a downstream protein called C3, or the C3 receptor on microglia in their mouse models, synapse loss did not occur.
Interestingly, the cells and proteins involved in synapse pruning — both normal and pathological — are essentially moonlighting. C1q is best known for initiating the complement cascade, an immunologic pathway for tagging unwanted cells and debris for clearance by other immune cells. Microglia are immune cells best known for engulfing and destroying invading bacteria.

Therapeutic potential
Hong notes that microglia and complement are already known to be involved in Alzheimer’s disease, but have been viewed as part of the plaque-related neuroinflammation that occurs in progressed stages of Alzheimer’s.
“Our study challenges this view and provides evidence that complement and microglia are involved much earlier in the disease process, when synapses are already vulnerable, and could potentially be targeted to preserve synaptic health,” says Hong.
This, of course, begs the question of what makes synapses vulnerable before complement or microglia get involved. But in the meantime, a human form of the antibody Stevens and Hong used to block C1q, known as ANX-005, is in early therapeutic development with Annexon Biosciences (San Francisco) and is being advanced into the clinic. The researchers believe it has potential to be used someday to protect against synapse loss in a variety of neurodegenerative diseases.
“One of the things this study highlights is the need to look for biomarkers for synapse loss and dysfunction,” says Hong. “As in cancer, if you treat people at a later stage of Alzheimer’s, it may already be too late.”
Complement, microglia and beta-amyloid
Much of the Alzheimer’s field is currently focused on beta-amyloid, the protein found in the plaque deposits that accumulate in the brain. The new research doesn’t question beta-amyloid’s role — in fact, together with co-author Dennis Selkoe, MD, at Brigham and Women’s Hospital, Stevens and Hong showed that beta-amyloid, C1q and microglia work together to cause early synapse loss.
Beta-amyloid in its oligomeric form (multiple unit strung together) was already known to be toxic to synapses even before it forms plaques. “We found that in the early stage, beta-amyloid oligomers require complement to be toxic,” Stevens says. The converse was also true: microglia engulfed synapses only when oligomeric beta-amyloid was present.
Annexon co-founders Ben Barres, MD, PhD, and Arnon Rosenthal, PhD, are coauthors on the paper. Barres, Rosenthal and Stevens are minor shareholders of Annexon LLC and Stevens is a member of the company’s Scientific Advisory Board. The study was funded by Edward R. and Anne G. Lefler Fellowship, Coins for Alzheimer’s Research Trust, the Fidelity Biosciences Research Initiative (F-Prime), JPB Foundation and the National Institutes of Health, the National Institute of Neurological Disorders and Stroke and the National Institute on Aging.
Related Posts :
-

The thalamus: A potential therapeutic target for neurodevelopmental disorders
Years ago, as a neurology resident, Chinfei Chen, MD, PhD, cared for a 20-year-old woman who had experienced a very ...
-

A surprising link between Crohn’s disease and the Epstein-Barr virus
Crohn’s disease, a debilitating inflammatory bowel disease, has many known contributing factors, including bacterial changes in the microbiome that ...
-

Could peripheral neuropathy be stopped before it starts?
An increase in high-fat, high-fructose foods in people’s diets has contributed to a dramatic increase in type 2 diabetes. This, ...
-

Status epilepticus: What’s changed, what to know, and a global perspective
Status epilepticus, or a prolonged seizure lasting more than five minutes, is a rare complication of epilepsy and a medical ...