From aerospace to the OR: 3D modeling improves surgical planning by revealing details of patients’ hearts
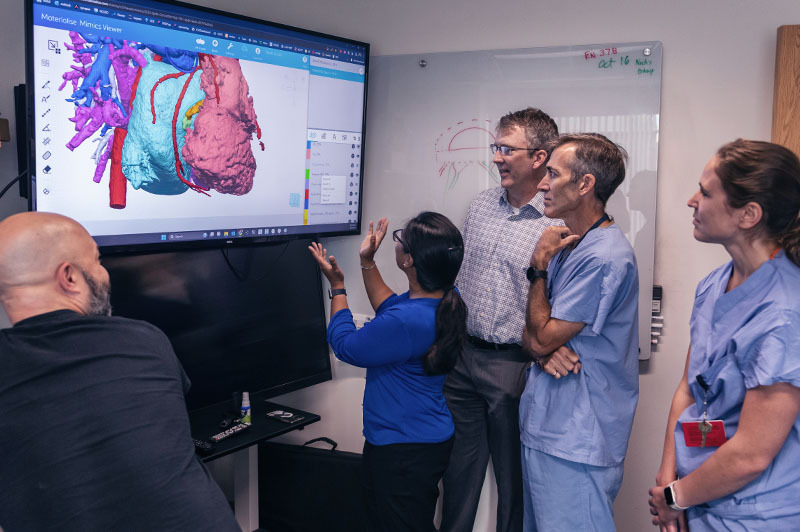
One of the most important tools for complex heart surgeries at Boston Children’s isn’t even in the operating room.
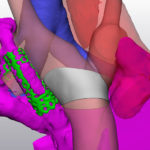
For years, heart surgeons couldn’t see the complete extent of a patient’s condition until a procedure started, forcing them to rely on experience, diagnostic imaging, and other information to plan surgery. Now, 3D modeling allows them to capture a comprehensive view of a child’s heart before surgery and plan the most effective repair procedure.
“People think in 3D, so to see the heart this way before surgery is transformational,” says David Hoganson, MD, a cardiac surgeon and co-leader of the team behind the modeling. “You can see the details of the heart within the pumping chambers and the vessels around the heart. That lets us better understand defects and possible repair options. Knowing with near-certainty what to expect eliminates guesswork in the operating room.”
3D models of a patient’s heart helped surgeons prepare a detailed plan for a mitral valve repair.
Combining experience and modeling
Heart surgery requires an understanding of physics. So it wasn’t all that surprising when, a few years ago, cardiac surgeon Christopher Baird, MD, asked the Cardiac Surgery engineering team to help with a procedure. Within hours, they calculated the ideal size of a graft tube that replaced damaged portions of a child’s aorta. “That opened the door to the surgeons now often asking us to predict, with numbers, what they do based on experience,” recalls Peter Hammer, PhD, a staff scientist and co-leader of the team.
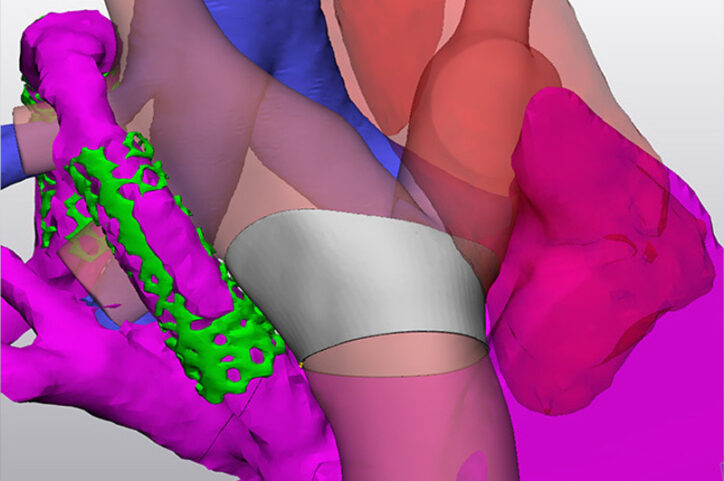
That work took a significant step forward when the team incorporated 3D modeling software that’s used by aerospace and automotive engineers into their calculations. In other words, simulation and engineering tools used to develop airplane wings could now illustrate how to treat complex heart disease. One of the earliest cases run through the software let them see the precise size and layout of a complex reconstruction of an 18-year-old’s heart. That perspective, combined with computer simulations of the teen’s circulation, helped them shape the design of a form-fitting cuff to repair his heart.
Finding the right 3D angle for a newborn’s surgery
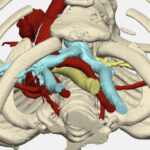
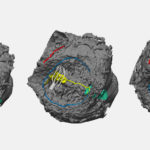
The team has since created more than 800 patient-specific 3D models. They create a model by uploading a still frame of a patient’s cardiac CT scan or MRI to the software. Or a bunch of those images can be stacked to create a movie that shows the heart beating, says physician scientist Reena Ghosh, MD, one of the team members who creates the models.
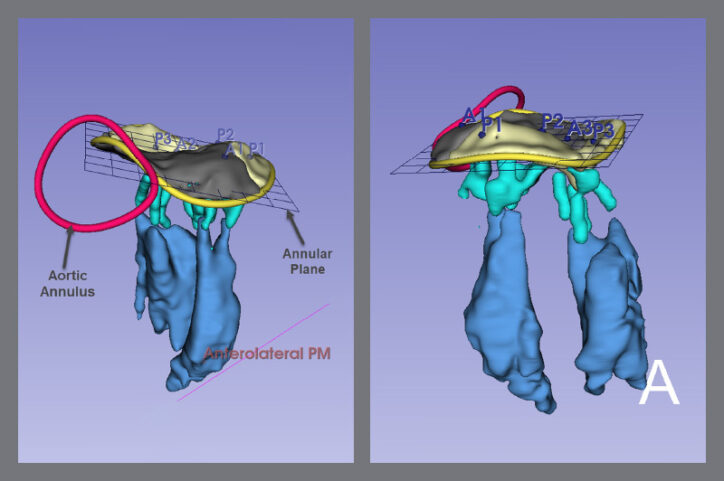
Modeling has enhanced surgical preparation for complex cases that involve congenital heart defects (CHDs) such as aortic arch anomalies, vascular rings, ventricular septal defects (VSD), tetralogy of Fallot, atrioventricular (AV) canal defects, and double outlet right ventricle (DORV).
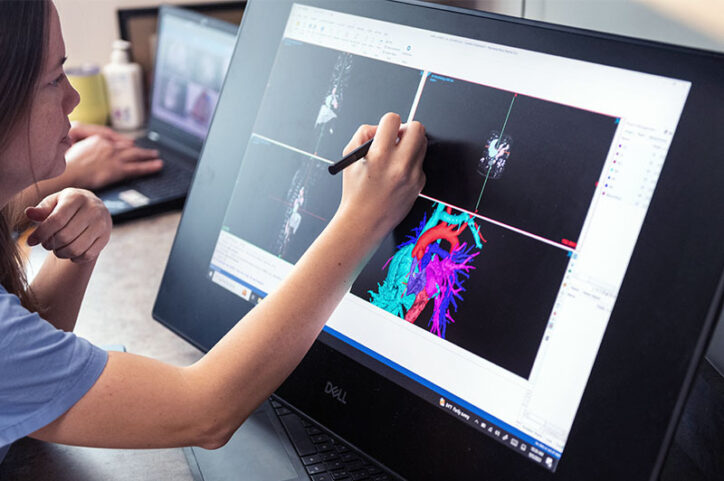
Recently, a model helped Hoganson treat a five-pound newborn who had a complex case of aortic arch narrowing.
The model offered a view of every part of her heart and aorta, from any angle. It also categorized each part as a separate visual component, meaning any one of them could disappear from the screen with a mouse click. This option unraveled the tightly-woven elements of her small heart and let the team understand which approach would work best.
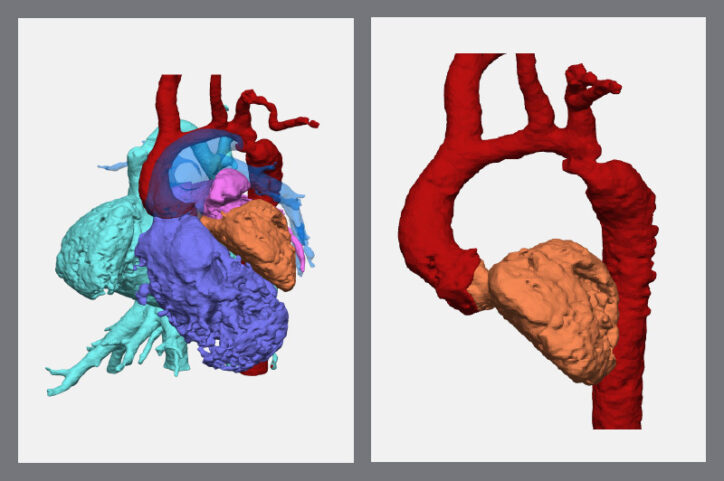
After they made a pulmonary artery disappear from the screen, they could see the transverse arch of her aorta was narrow. That weakened the left ventricle and limited the pumping of oxygenated blood throughout her body.
With those perspectives, Hoganson and his surgical team had a precise plan that let them reconstruct the aorta and restore function to the ventricle. The newborn is now home and thriving.
New viewpoints to help surgeons and parents
A surgeon’s experience of knowing how to approach treatment still matters, Hoganson says, but 3D modeling lets them create specific treatment plans for each individual. “It’s a difference-maker to be able to say, ‘For this child, the valve leaflet needs to be lengthened by four millimeters here and two millimeters there so we can create a perfectly-shaped patch.’”
Such calculations allow surgeons to preserve as much of a patient’s heart anatomy as possible and limit implanted prosthetic materials. The 3D models also show the heart as it appears when beating. During most complex procedures, the heart isn’t beating; a patient’s heart is temporarily stopped by diverting blood to a heart-lung machine so surgeons can safely operate. “With those calculations, they will know what to do when the heart’s in a flaccid state so they can restore it to dimensions for when it beats again,” Hammer says.
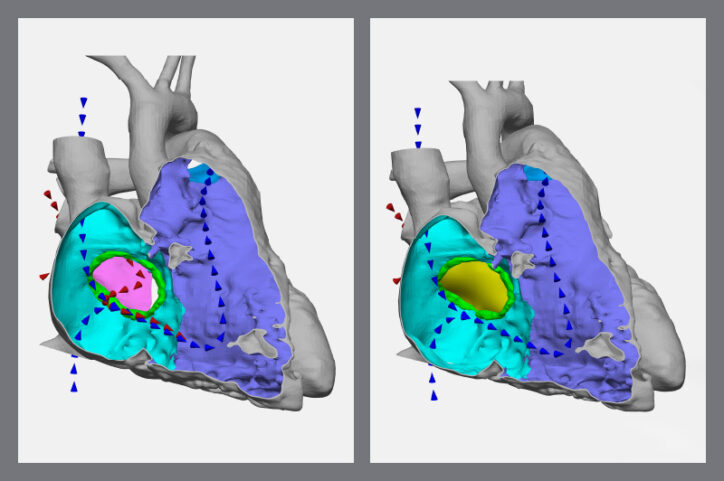
The 3D models have also helped parents visualize the extent of their child’s condition, rather than learning about it mostly through words. The models can show parents blood flow through the heart before and after the repair and where patches will be placed. “They’re very eager to see what their child’s heart looks like,” Ghosh says, “and have better information to understand the surgical plan.”
The 3D future looks even brighter
While the team is excited by the gains they’ve made, they believe they haven’t yet tapped 3D modeling’s potential.
Ghosh says it’s a matter of time before the team can have modeling replicate what a heart will look like after surgery, which would help determine the best option before a procedure. That would advance the repair of valves, the parts of the heart that move the most and require an extensive level of surgical calculations, she says.
“The innovation and use of 3D modeling have been truly transformative in the way we can plan and perform complex surgeries,” Baird says. “Not only does it save valuable time when a patient’s heart is stopped during surgery, but it also allows us to be more accurate and precise.”
Learn more about the Department of Cardiac Surgery or refer a patient to the Benderson Family Heart Center.
Related Posts :
-

Utilizing engineering tools from the aerospace industry to repair hearts
When an 18-year-old patient from North Carolina recently presented at Boston Children’s Heart Center with an enlarged right atrium ...
-

Finding a way to help newborns who can't immediately have heart treatment
Newborns with complex congenital heart defects (CHD) and pulmonary overcirculation often need treatment as soon as possible. Unfortunately, ...
-

Reconstructing a chest wall, one virtual step at a time
It takes a village of clinicians and engineers to reconstruct a chest wall. It also takes a lot of 3D ...
-

Two new approaches to identifying conduction tissue
Conduction cells in the heart are responsible for initiating contraction of the heart muscle. The inability to properly identify the ...