Two new approaches to identifying conduction tissue
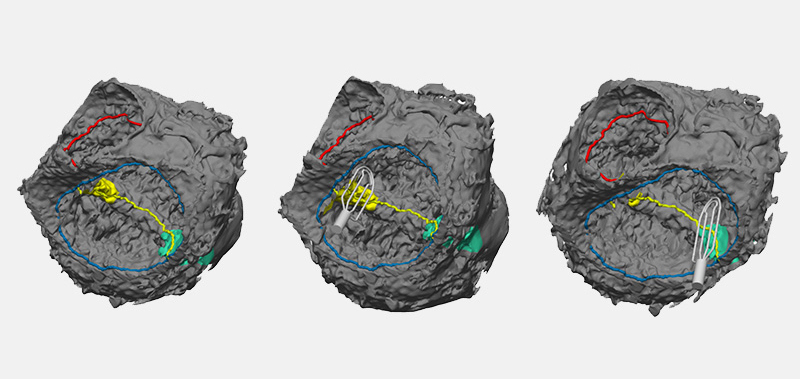
Conduction cells in the heart are responsible for initiating contraction of the heart muscle. The inability to properly identify the location of conduction tissue in patients with congenital heart defects during heart surgery can lead to post-operative conduction abnormalities such as heart block or conduction delays. This necessitates the need for pacemakers, which leads to additional operations and risk of morbidity. And for the children who require a pacemaker, it’s a significant quality-of-life issue.
This problem has led clinicians in the Heart Center at Boston Children’s Hospital to develop two novel approaches to locate conduction tissue during surgery. The first uses real-time fiberoptic confocal microscopy (FCM) inside the heart, while the second utilizes electrophysiology (EP) tools adapted from the EP lab to measure electrical signals in the heart.
Confocal microscopy
“Fiberoptic confocal microscopy is a low-power laser light that comes through a fiberoptic microscope and shines at the tissue, enabling detailed imaging of tissue microstructure,” says Aditya (AK) Kaza, MD, cardiac surgeon and director of the Neonatal Cardiac Surgery Program at Boston Children’s. “Although the microscope was developed to look for cancer in the esophagus and stomach, we adapted it to look at conduction inside the human heart, using a laser source and image detectors.”
Next, Kaza started looking for a fluorescent dye to clarify the specific architecture of the cardiac tissue. After some initial trials, his team landed on fluorescein, which was already approved by the FDA for other applications, but using a concentration of 1000th of the recommended dose.
After experimenting with the FCM system using fluorescein in animal models, Kaza began a trial in 2018 to test the technique in six patients with simple atrial septal defects. He found it worked well. “We were initially concerned about how difficult it would be to steer the microscope through the human heart, but it’s actually pretty flexible,” he says.
Kaza says the imaging takes two to five minutes in the operating room (OR) after the heart is stopped and the bypass machine is on. “I apply fluorescein on the surface of the heart and use the probe to image the heart to find the conduction tissue. The image comes up on the monitor so I can capture the recording. This allows me to go back and re-check the area if I need to.”
He is currently enrolling patients with simple heart defects, such as ventricular septal defects, tetralogy of Fallot, and atrioventricular canal defects in a prospective, randomized clinical trial. He is also preparing another single center clinical trial to look at the role of confocal microscopy in more complex repairs, such as biventricular repairs and L-loop transpositions. This clinical trial is funded by the National Heart, Lung, Blood Institute at the National Institutes of Health.
“We’re looking to see if this system causes less risk of residual defects over the traditional way. If it’s successful, it has the potential to become a part of every open-heart surgery we do.”
The electrophysiology approach
While conduction tissue can vary from heart to heart, it’s even more difficult to map in patients with complex heart defects, such as those undergoing a biventricular repair, because their anatomy is so unpredictable.
“We noticed the incidence of heart block was about 15 percent in patients with biventricular repairs, which was higher than we wanted,” says Elizabeth DeWitt, MD, a cardiologist in the Electrophysiology Service at Boston Children’s. To look for ways to reduce these numbers, DeWitt and her colleague Edward O’Leary, MD, began working with cardiac surgeon Eric Feins, MD, using EP tools to locate the conduction system in these patients’ complex hearts.
“We decided to use a catheter from the lab in the OR, but instead of putting it through the groin, we had the surgeon place it onto the heart directly,” says DeWitt. “From there we began to develop a crude understanding of where conduction was.”
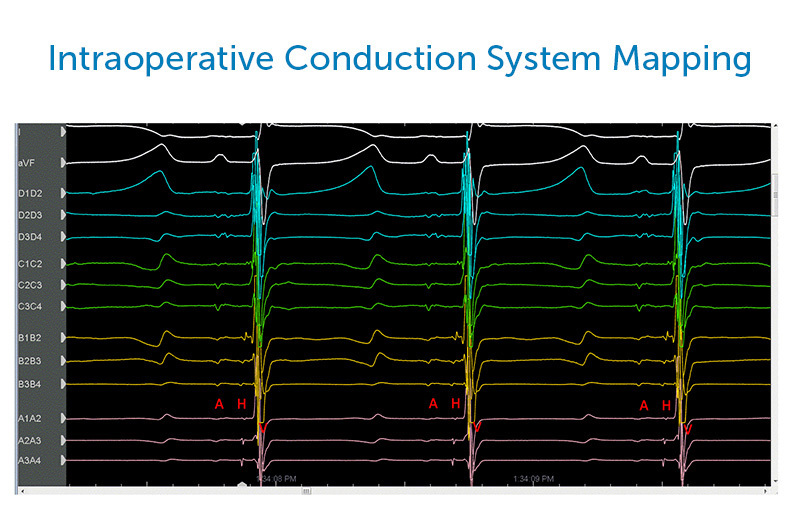
Over the past year, DeWitt, O’Leary, and Feins have further refined their process, using various types of catheters and exploring different ways to visualize conduction in the OR. Their technique works while the heart is still beating on bypass.
“As the surgeon moves the catheter around to different areas of the heart, we’re looking for specific signals at the His bundle,” says DeWitt. “Then we communicate back and forth with the surgeon about where we see conduction based on that information.”
From these cues, the surgeon can form a general image of where the conduction system is located and avoid injuring it. Although there is currently no actual visual “map” for the surgeon to follow, as there is in Kaza’s technology, the team is continuing to explore modifications to help surgeons better visualize conduction.
“We’re hoping over the next several months to create a mapping system like we use in the EP lab, where you create a shell of the heart and overlay the electrical activity on top of it, so we can create a 3-D image that the surgeons can reference back to,” says DeWitt.
Mapping the heart
Creating better maps of the conduction system is a common goal of both teams. “We are currently putting together a consortium where we obtain the original hearts from heart transplant patients and do a complete cartography mapping of the conduction system,” says Kaza. “We’re using this information to develop an open source database that anybody in the world can use to help guide them in repairs.”
DeWitt, Feins, and OLeary have also been working to map the conduction system of hearts, using 3-D models developed by cardiac surgeon David Hoganson, MD, and his group of engineers. “We’re annotating where we’ve found conduction on those models, not only so we can keep that record for that individual patient for potential future surgery, but also so we can create an atlas of where we’ve found conduction on different types of hearts,” says DeWitt.
A collaborative future
While the two techniques are not currently used together, the clinicians envision a future in which they will work collaboratively to help create a more detailed look at conduction.
“I see us using both technologies in a complementary way,” says Feins. “Using the EP techniques, we’re able to zero in on the conduction system in about 10 seconds and determine the region it’s in, while the heart is still beating. Then, once you’ve stopped the heart and are ready to begin the actual repair, you bring in AK’s device to make sure you’re staying away from a very precise area.”
Ultimately, Kaza envisions using these technologies in all heart surgery patients. “I think we’re going to look back five or ten years from now and won’t be able to imagine that we were ever performing surgery without technology that helps us localize conduction tissue.”
Learn more about the Heart Center.
Related Posts :
-

Unique data revealed just when Mickey’s heart doctors could operate
When Mikolaj “Mickey” Karski’s family traveled from Poland to Boston to get him heart care, they weren’t thinking ...
-

Past patient outcomes could help single-ventricle surgery decisions
When considering whether a child who has a single-ventricle heart defect would benefit more from biventricular repair&...
-

Every heart has a story. See how we care for them all.
It’s easy to think of the heart as a common denominator — everyone has one — but no two hearts are ...
-

It’s all in the PV loops: New analytical model could improve circulation assessments before heart surgery
The double-switch operation corrects the congenital reversal of the heart’s ventricles and its two main arteries. It’...





