First sharp images reveal structure of key inflammatory protein
After decades of attempts by the scientific community, researchers have now provided the first clear look at a protein implicated in a vast array of inflammatory conditions.
The finding, published recently in Nature, lifts a blindfold that has hampered scientists’ ability to intervene when the immune system overreacts to perceived threats.
The protein, known as NLRP3, alerts immune cells when it senses invading viruses and bacteria and other dangers. The cells then marshal defenses, including the assembly of inflammasomes, molecules that tell compromised cells to self-destruct to prevent the threat from spreading.
But sometimes NLRP3 sounds an unnecessary or disproportionate alarm. The proteins may call for assaults on arterial plaques or uric acid crystals or healthy tissues or fail to shut off after mobilizing an appropriate immune response.
Few proteins have been implicated in as many diseases as NLRP3.
The results: autoimmune diseases; chronic inflammation that contributes to cardiovascular disease, type 2 diabetes, gout, and severe nonalcoholic fatty liver disease; neuroinflammation such as in Alzheimer’s and Parkinson’s diseases; and sepsis, a life-threatening systemic overreaction to an infection.
“Few proteins have been implicated in as many diseases as NLRP3,” says study co-senior author Hao Wu, PhD, a professor in Harvard Medical School’s Blavatnick Institute and a member of the Program in Cellular and Molecular Medicine at Boston Children’s Hospital.
NLRP3: New target for inflammation?
By capturing NLRP3’s detailed, three-dimensional molecular structure and revealing how another protein allows it to activate, Wu and colleagues have laid the foundation for designing drugs that bind to NLRP3 and muffle its alarm.
“There has been huge interest in developing anti-inflammatory drugs that stop NLRP3 activation,” said Humayun Sharif, PhD, a postdoctoral fellow in the Wu lab and co-first author of the study. “Understanding its structure will help by providing a blueprint.”
The discovery should also allow researchers to determine whether any existing drugs act on NLRP3 and tackle diseases that arise from mutations in the gene that produces NLRP3.
Not least, it will help biologists dig further into the basic mechanisms of inflammasomes.
Sticky situation
The main reason scientists were stymied all those years was that the instruments that could reveal the structure of NLRP3 required clean, individual proteins. But NLRP3 insists on clumping together.
Co-first author Li Wang, PhD, a former Wu lab postdoctoral researcher, finally overcame this hurdle by conducting some clever protein engineering that convinced NLRP3 to stay single.
I couldn’t sleep at night. I would see proteins move when I closed my eyes.
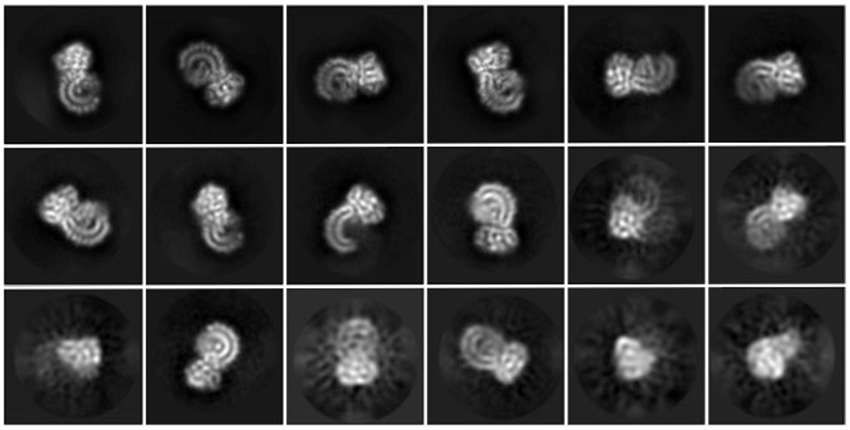
The team then used cryo-electron microscopy, or cryo-EM, to snap high-resolution pictures of NLRP3. Partnering with the lab of co-senior author Youdong Mao, PhD, at Peking University, including co-first author Wei Li Wang, PhD, the researchers quintupled the number of images they were able to obtain. In all, they took 2 million pictures and analyzed only the best 100,000.
The results delivered two surprises.
Protein puzzle pieces
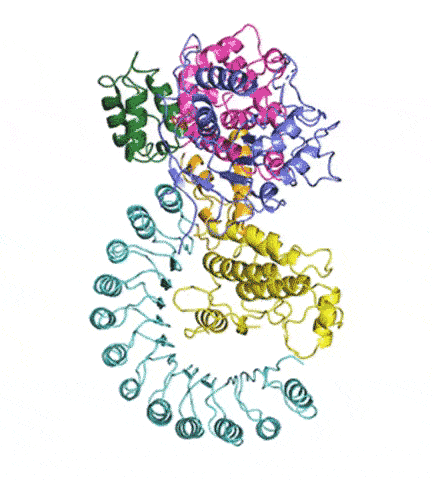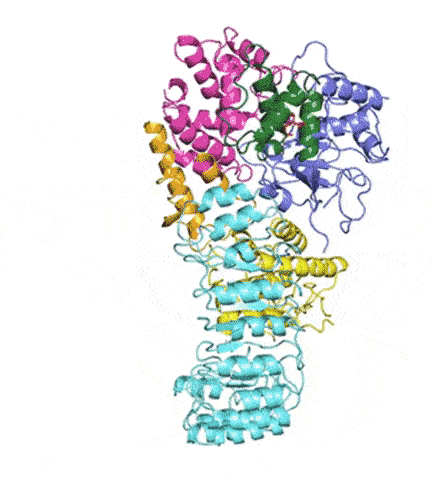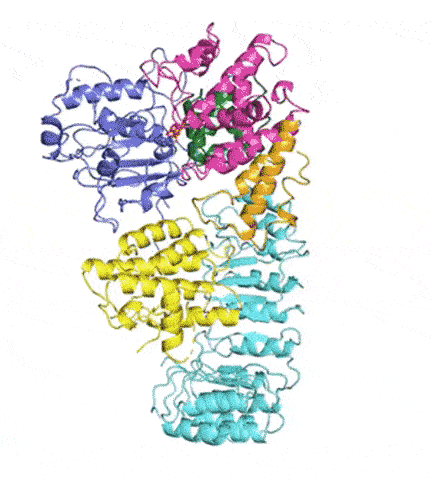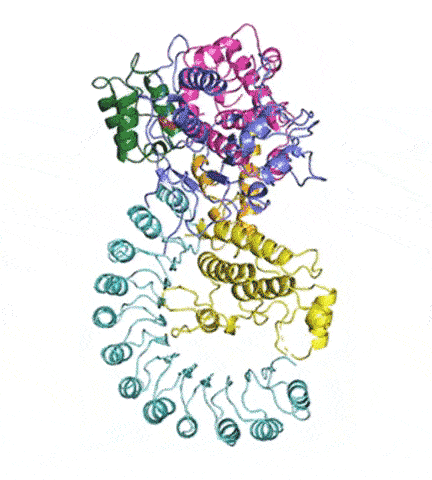
At last, NLRP3’s shape became clear. The researchers liken it to an earring, with a stud connected to a C-shaped hoop.
Next came the question of how NLRP3 goes from its dormant to its alarm-blaring state. Day after day, Sharif played a kind of protein Tetris on the computer to figure out whether the scientific community’s top candidate, the protein NEK7, could lock on to NLRP3’s unique shape and activate it.
“I couldn’t sleep at night,” said Sharif. “I would go home and see proteins move when I closed my eyes.”
At last, NEK7 slotted into place.
Although it’s not yet clear which other players may be involved, Wu says the work “settled some longstanding questions in the field about whether NEK7 is important in inflammasome activation.”
Simulations showed that NEK7 nestles into the cup of NLRP3’s C-shaped hoop. Eleven NLRP3-NEK7 combos then form a ring, with the studs in the middle and the hoops out.
“NEK7 helps the components of the ring come together,” Sharif said. “We never would have expected that. Seeing that was one of the coolest parts of this project.”
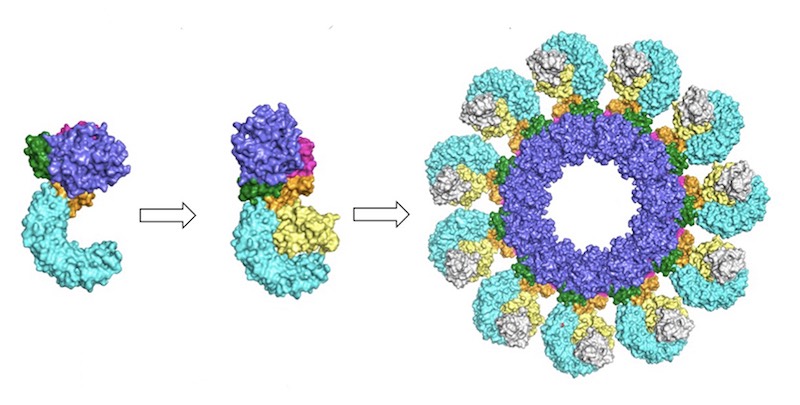
NEK7 was previously known to be involved in mitosis, or cell division. Since it can’t bind to NLRP3 and to mitosis-related molecules at the same time, the researchers suspect that dividing cells can’t sound the immune alarm and alarm-blaring cells can’t divide.
Long-term triumph
The biggest surprise, though, said Wu, was that the team solved the structure at all.
The work was so difficult and painstaking that it took three generations of trainees in her lab to complete the project.
“There is a joke that the lawn outside our building is haunted by the ghosts of past postdocs who worked on this project,” said Sharif.
“It’s really satisfying that we got an answer,” said Wu. “I feel like this means that it doesn’t matter how difficult a problem is; if you put in enough effort, you’ll get it.”
The fine print
Wu lab members who co-authored the paper include Venkat Giri Magupalli, Liudmila Andreeva, Qi Qiao, and Arthur Hauenstein. Other co-authors were Gabriel Núñez of the University of Michigan Medical School and Zhaolong Wu of Peking University. Mao and Wei Li Wang are also affiliated with the Department of Microbiology in the Blavatnik Institute at Harvard Medical School and the Dana-Farber Cancer Institute.
Wu is co-founder of SMOC Therapeutics, a company developing drugs for diseases driven by the innate immune system. Hauenstein and Li Wang are employees.
This work was funded in part by the U.S. National Institutes of Health (grants DP1HD087988, R01AI124491 and R01AI06331), Intel Corporation, Thousand Talents Plan of China, National Natural Science Foundation of China (grant 11774012), and Natural Science Foundation of Beijing City (grant Z180016).
This post originally appeared on Harvard Medical School’s news site.
Related Posts :
-

Navigating school with a neuroimmune condition
Fifteen-year-old Sarah had been challenging her dad to card games all week — and on Saturday, she finally beat him. It ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

No limitations: How Flora found answers for MOG antibody disease
Flora Ringler’s fifth birthday didn’t turn out as she had hoped. She and her family were vacationing in ...
-

‘They never stopped trying to figure out what was happening’: RyennAnne’s encephalitis journey
When 5-year-old RyennAnne Hurst developed a bad sore throat last summer, her doctor thought she might have strep and prescribed ...





