Microvillus inclusion disease: From organoids to new treatments

Microvillus inclusion disease (MVID) is a rare type of congenital enteropathy in infants that causes devastating diarrhea and an inability to absorb food. Infants can lose liters of fluid a day, become severely dehydrated, and stop growing. There is no specific treatment.
“Until about 10 years ago, 50 percent of kids with MVID would die before age 2,” says Jay Thiagarajah, MD, PhD, who co-directs the Congenital Enteropathy Program at Boston Children’s Hospital. “Because of advances in parenteral nutrition, it has become a manageable disease. But children need parenteral nutrition every day and are still often in the hospital for dehydration and infections.”
Several years ago, Thiagarajah began investigating MVID’s root cause as a way of finding better treatments. Studying patient-derived organoids, which function like mini small intestines, his team has uncovered two promising treatment leads. One will soon enter a clinical trial.
Creating organoids with MVID
Like other congenital enteropathies, MVID disrupts the structure and function of the epithelial cells that line the intestine. In particular, MYO5B — the genetic cause of MVID — is known to be important for maintaining the microvilli, the finger-like projections that increase the intestine’s surface area to maximize nutrient absorption. In MVID, microvilli are either absent or malformed and unable to absorb.
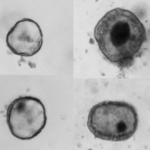
To understand how mutations in MYO5B lead to disease, Thiagarajah and his colleagues obtained intestinal biopsy tissue from a small handful of patients and isolated the intestinal stem cells. They then used these cells to construct the organoids.
A little surprisingly, we found that the microvilli had improved structure and function, and some functions of sodium absorption were improved.”
“People have knocked out MYO5B in animal models, but no one had looked at patient-derived cells before,” Thiagarajah says. “No one could grow intestinal cells until recently because it’s so difficult to get these cells from patients. It’s a rare disease, and patients rarely have endoscopies after they’re diagnosed.”
The lab-made organoids showed the same pathology found in children’s intestines: loss of structurally intact microvilli. Their creation set the stage for exploring the disease process and potential treatments.
Defining MVID’s physiology
In studying the organoids, Thiagarajah and his colleagues discovered that dysfunctional electrolyte transport is the reason for the fluid loss in MVID. Specifically, intestinal cells lose the ability to absorb sodium and the water that travels with it. Meanwhile, chloride and fluid secretion remain normal. The net result is fluid loss.
“There was an obvious thing we could try: blocking chloride secretion,” says Thiagarajah. “It doesn’t fix the sodium absorption problem, but it could help with fluid loss.”
As reported in the Journal of Clinical Investigation, an existing chloride channel blocker, crofelemer, reduced chloride (and water) secretion in the organoids. Currently, crofelemer is approved for adult diarrhea caused by HIV or chemotherapy. However, the team recently obtained FDA permission to test crofelemer in a clinical trial sponsored by the manufacturer.
Boston Children’s will be the first site, but given the rarity of MVID, the trial will eventually expand to sites all over the world. It is hoped to open this spring or summer.
Correcting MVID at its source
The organoid research also revealed a possible way to reverse the root cause of MVID. Previous studies in a mouse model, based on knockout of MYO5B, had suggested that a gamma-secretase inhibitor could prevent loss or malfunction of the microvilli.
“We tested to see if gamma-secretase inhibition could rescue microvilli in our organoids,” Thiagarajah says. “A little surprisingly, we found that the microvilli had improved structure and function, and some functions of sodium absorption were improved. These cells had been pretty screwed up before.”
Because gamma-secretase inhibitors cause multiple side effects, the team is now searching for a more specific pathway to target to restore the microvilli. A genome-wide transcriptomic analysis of MVID organoids versus healthy organoids has provided some interesting leads to pursue.
“The possibility of reversing the disease itself, rather than just its symptoms, is for me the most exciting part,” says Thiagarajah.
Learn how we care for children in the Congenital Enteropathy Program.
Related Posts :
-

A perfect genetic hit: New gene mutation implicated in rare congenital diarrhea
When the 1-year-old boy arrived from overseas, he was relying on total parenteral nutrition — a way of bypassing the digestive ...
-

A new approach to C. diff? Targeting the inflammation, not the bacteria
Clostridium difficile (C. diff) intestinal infections can cause severe, debilitating diarrhea in patients who are hospitalized or on immunosuppressive therapies. ...
-

Going 'all in' for Khori: New hope for congenital enteropathy
Khori LeBlanc is “one of the sassiest and sweetest kids you’ll ever meet,” says her mom, Bryanna Black. Her ...
-

Saving Laila: Family travels from Egypt for answers about rare genetic condition
When Aya Hendawy got off the plane that had brought her from Egypt to Boston, she didn’t linger in ...