Intestine chip models gut function, in disease and in health
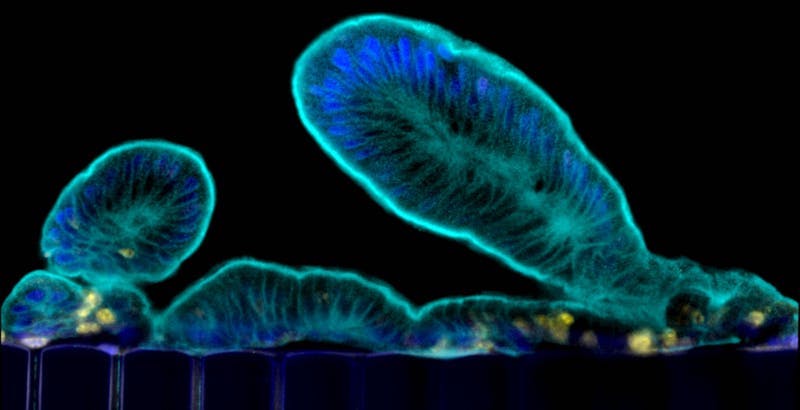
The small intestine is much more than a digestive organ. It’s a major home to our microbiome, it’s a key site where mucosal immunity develops and it provides a protective barrier against a variety of infections. Animal models don’t do justice to the human intestine in all its complexity.
Attempts to better model human intestinal function began with intestinal “organoids,” created from intestinal stem cells. The cells, from human biopsy samples, form hollowed balls or “mini-intestines” bearing all the cell types of the intestinal lining, or epithelium. Recently, intestinal organoids helped reveal how Clostridium difficile causes such devastating gastrointestinal infections.
But while organoids have all the right cells, they don’t fully replicate the environment of a real small intestine. Real intestines are awash in bacteria and nutrients, are fed by blood vessels and are stretched and compressed by peristalsis, the intestines’ cyclical muscular contractions that push nutrients forward.
Efforts to recreate that environment led to the Intestine Chip. An early version, created by the Wyss Institute for Biologically Inspired Engineering, cultured cells from a human intestinal tumor cell line. The developers seeded these cells into a microfluidic device with miniaturized channels, simulating natural cell layers, and applied mechanical forces to mimic blood flow and peristalsis. The resulting model grew villi — the nutrient-absorbing, finger-like projections of the intestinal epithelium — and enabled insights into how blood flow and peristalsis affect intestinal function.
Yet, the Chip’s tumor-derived intestinal cells don’t fully recapitulate the physiology of the normal intestinal epithelium, including the formation of different cell types to handle the intestine’s various jobs. Nor can they model medical conditions, such as inflammatory bowel disease.
Back to the future: Chips meet organoids
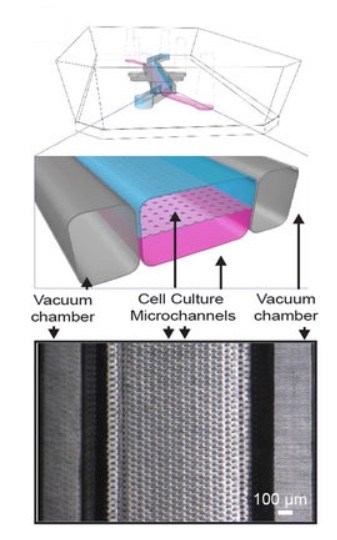
So, in the next phase, the Wyss team collaborated with the laboratory of David Breault, MD, PhD at Boston Children’s Hospital, to build an organoid culture step into their procedure. Breault, also a member of the Harvard Stem Cell Institute, has been working with intestinal stem cells for a decade, and more recently on organoids.
As described last week in Scientific Reports, the team took organoids created from patient biopsies of the duodenum and seeded the cells onto organ chips. The chip’s “epithelium” channel, bearing the organoid cells, mimics the intestinal lining and surrounds a hollow cavity (lumen) through which nutrients are flowed. An adjacent “vascular” channel bears surrogate blood vessels, derived from intestine-specific vascular endothelial cells.
With the help of a specialized culture medium and mechanical stimulation, the organoids’ intestinal stem cells differentiated into various specialized cell types. These included nutrient-digesting and absorbing enterocytes, mucus-producing goblet cells, hormone-secreting enteroendocrine cells and Paneth cells, which secrete antimicrobial peptides and proteins and help rejuvenate the epithelium. The researchers also saw evidence of digestive enzymes and strong barrier function.
The best of both worlds
By collecting fluids flowing through the lumen, researchers can quantify nutrient digestion, mucus secretion and establishment of intestinal barrier function over time. As culture conditions like flow and mechanical stretch are modified, they can measure biochemical, genetic and cellular responses.
This could make the new chip a useful research tool for studying normal intestinal metabolism and nutrition, infection, drug pharmacokinetics, the influence of the microbiome and more, says Breault. And because it’s built from cells from individual patients, it could be used to model disease processes.
There’s also room for enhancement.
“The Chip-monolayer approach may facilitate the incorporation of other relevant cell types such as microbes, mesenchymal and immune cells, and as such, will bring the field even closer to a tractable experimental system that faithfully recapitulates human intestinal epithelial physiology,” write Breault and Camilla Richmond, MD, an attending in pediatric gastroenterology at Boston Children’s and a coauthor on the paper.
Donald Ingber, MD, PhD, founding director of the Wyss Institute and a member of the Vascular Biology Program at Boston Children’s Hospital, was co-senior author on the paper. Magdalena Kasendra, PhD, a former postdoctoral fellow on Ingber’s team and now at Emulate, Inc. in Boston, was co-first author, along with Alessio Tovaglieri, MS, a former member of the Breault Lab and now a PhD student at the Wyss Institute.
Details in the paper and this press release from the Wyss Institute.
Related Posts :
-

Team spirit: How working with an allergy psychologist got Amber back to cheering
A bubbly high schooler with lots of friends and a passion for competitive cheerleading: On the surface, Amber’s life ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...
-

Genetic causes of congenital diarrhea and enteropathy come into focus
Congenital diarrheas and enteropathies are rare and devastating for infants and children. Treatments have consisted mainly of fluid and nutritional ...
-

Genomic sequencing transforms a life: Asa’s story
Asa Cibelli feels like he’s been reborn. The straight-A middle schooler plays basketball and football, does jiu jitsu, is ...





