Groundbreaking research helps advance treatment of rare, fast growing brain tumor
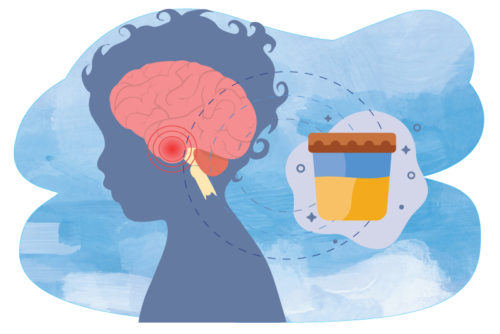
Researchers from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center recently found that levels of a specific protein detected through a patient’s urine can track a tumor’s size and responsiveness to treatment in children with diffuse intrinsic pontine gliomas (DIPG). This discovery helps steer the course for more innovative and less invasive treatment options.
Confronting the challenges of DIPG
DIPG affects 150 to 300 children in the United States every year and poses a greater than 98 percent mortality rate within five years of diagnosis. This unsettling statistic is largely due to the clinical challenge DIPG presents. Surgery isn’t a strong possibility because of the invasive nature of the tumor, and there are not yet effective chemotherapeutic options. Currently, radiation is the primary approach to treatment, but it only provides an average survival of a few months.
Because of these challenges, there is a critical need to develop ways to detect DIPG, monitor treatment effectiveness, and identify mechanisms that control key pathological features of this kind of tumor.
Thanks in part to a donation from Love Your Melon — an apparel brand committing 50 percent of its profit to organizations that fight pediatric cancer — a team led by Edward Smith, MD, director of cerebrovascular neurosurgery and co-director of the Cerebrovascular Surgery and Interventions Center at Boston Children’s Hospital, is working on groundbreaking research to meet these needs.
Urinary biomarkers are charting a new course
“Urinary biomarkers help us avoid as much as possible the invasive and uncomfortable standard practice of poking children in the head,”
Ed Smith, MD
“Ultimately, what we’re trying to do is find better ways to detect these types of tumors and follow them by using urine,” he explains.
To do this, Dr. Smith and his team took part in the first successful use of urinary biomarkers for DIPG in a national trial, which determined that the netrin-1 protein is a reliable biomarker — or measurable indicator — of DIPG.
Netrin-1 is produced by the NTN1 gene. The NTN1 gene’s function in the human body hasn’t been entirely determined, but it is thought to be involved in cell migration.
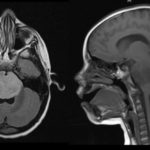
“Urinary biomarkers help us avoid as much as possible the invasive and uncomfortable standard practice of poking children in the head,” says Dr. Smith. Currently, the standard method of diagnosis for DIPG is an MRI as well as a biopsy.
Other advantages of biomarkers include:
- non-invasiveness (no need for sedation or anesthesia)
- reduced risk inherent in surgical procedures
- lower cost than standard diagnostics
- ease and frequency of sampling (no needles or spinal taps and less travel involved for patient and family to undergo tests)
“We’re really trying to find unique ways to target the brain cancer cells and kill them to stop them from invading normal, healthy brain cells,” Dr. Smith says. “We’re working hard to help so many wonderful kids.”
As part of one of the world’s largest pediatric glioma treatment programs, the Boston Children’s Department of Neurosurgery provides extensive expertise in treating all types of gliomas, including DIPGs.
Learn more about how our pediatric brain tumor specialists maximize outcomes for patients.
Related Posts :
-

Solving the DIPG puzzle a single cell at a time
For more than 15 years, pediatric neuro-oncologist Mariella Filbin, MD, PhD, has been on a scientific crusade to understand DIPG (...
-

Research opens a window into understanding deadly brain tumors
Formerly known as diffuse intrinsic pontine gliomas, diffuse midline gliomas (DMGs) are highly aggressive tumors found in the midline of ...
-

From our labs and clinics: 10 research advances in 2021
Pediatric medicine at Boston Children’s Hospital rests on a strong base of discovery science. But it can take decades ...
-

Guidance for assessing treatment response in pediatric brain tumors
Assessing patients’ response to cancer therapy can be challenging, especially in neuro-oncology. Generally, we assess treatment response by a change ...