Research opens a window into understanding deadly brain tumors
Formerly known as diffuse intrinsic pontine gliomas, diffuse midline gliomas (DMGs) are highly aggressive tumors found in the midline of the brain. Their prognosis is very poor, in part because they don’t respond well to treatments such as chemotherapy and radiation.
Now, research by Mariella Filbin, MD, PhD, and others in the Brain Tumor Center at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center has uncovered new details about how these rare tumors develop and grow that could someday inform specialized treatment options.
Differences between pediatric and adult DMGs
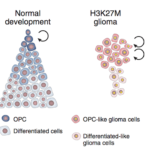
In a study published in Nature Genetics, Filbin and her colleagues looked for age and location-dependent differences in DMGs by measuring all the individual cell activities of thousands of cells in tissue samples from 50 children and adults with a Histone3-K27M mutation. The team found that pediatric tumors — as well as tumors lower in the brainstem and spinal cord in patients of all ages —contain more stem-cell like, immature cells. Immature cells can proliferate quickly and easily because of their similarity to normal stem cells, which might help explain the lethality and treatment resistance of DMGs in kids.
The researchers also noticed that adult tumors have more mesenchymal-like tumor cells, which can normally help healing damaged tissues but also aid tumor growth and progression in cancer. However, they didn’t detect any underlying genetic cause for this difference amongst different tumor cells. These findings suggest that cancer cells are shaped differently by normal brain cells around them across different age groups, and that kids and adults with DMGs might need different treatments, explains Filbin.
How tumors build a “wall” of protection
The researchers also discovered that cancer stem cells (the most immature cells) had formed “niches” by clumping together and surrounding themselves with a type of cancer cell that can shield and protect the growing cancer cells from immune attack.
“We now have a glimpse into how tumors visually organize themselves — walling off from the rest of the brain to prevent an immune attack and getting closer to cells that feed them,” says Filbin. Understanding the structure of the tumor helps researchers to think about new treatment approaches.
The work adds to another study by Filbin published earlier this year, which found that a family of proteins called the BRG1/BRM associated factor (BAF) chromatin remodeling complex plays a critical role in DMG tumor development, and can be targeted with novel chemical compounds designed at Dana-Farber/Boston Children’s.
“This is the first time we have been able differentiate DMG cells into non-dividing, less tumorigenic cells —not only genetically, but also chemically. We are now working with our chemists, especially Dr. Jun Qi, to modify these compounds so they can enter the brain at higher concentrations,” says Filbin. “I’m excited about the prospect of this class of drugs for brain tumors.”
Learn more about the Brain Tumor Center or refer a patient.
Related Posts :
-

Solving the DIPG puzzle a single cell at a time
For more than 15 years, pediatric neuro-oncologist Mariella Filbin, MD, PhD, has been on a scientific crusade to understand DIPG (...
-

Groundbreaking research helps advance treatment of rare, fast growing brain tumor
Researchers from the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center recently found that levels of a specific protein ...
-

Unlocking a treatment for diffuse intrinsic pontine glioma
Diffuse intrinsic pontine glioma (DIPG) is a highly aggressive and one of the most difficult-to-treat childhood tumors. It’s ...
-

Single-cell sequencing reveals glioblastoma's shape-shifting nature
Glioblastoma, a cancer that arises in the brain’s supporting glial cells, is one of the worst diagnoses a child ...