Exploring autism by way of three rare genetic disorders
Rajna Filip-Dhima, MS is a senior project manager for the Translational Neuroscience Center at Boston Children’s Hospital and for the Developmental Synaptopathies Consortium, which just received a new cycle of NIH funding.
Autism spectrum disorder (ASD) is now believed to affect 1 in 59 children in the U.S. Over the past five years, Boston Children’s Hospital has led a quest by ten medical centers to explore the underlying causes of ASD and intellectual disability by focusing on three rare genetic disorders with features of both: tuberous sclerosis complex (TSC), Phelan-McDermid syndrome (PMS), and PTEN hamartoma tumor syndrome (PHTS).
Despite being caused by genetic variants affecting different genes (TSC1 and TSC2 in the case of TSC, SHANK3 in PMS, and PTEN in PHTS), the three syndromes affect shared cellular pathways that influence the development of brain connections, or synapses. The long-term goals of the Developmental Synaptopathies Consortium (DSC), led by Mustafa Sahin, MD, PhD, are to better understand the pathophysiology of these rare disorders and to discover therapeutic strategies.
Developmental synaptopathies: Five years of findings
The DSC was formed through a five-year NIH grant establishing the Rare Disease Clinical Research Network. To date, the DSC has had many successes, which we plan to extend with a new cycle of funding. Among our achievements to date:
- Creation of an infrastructure enabling researchers in multiple disciplines to collaborate at ten institutions throughout the U.S.
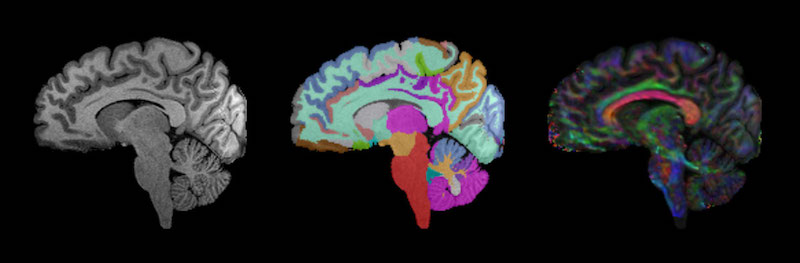
- Analysis of hundreds of MRI scans of participants with TSC, PMS, and PHTS, through the expertise and leadership of Simon Warfield, PhD, director of the Computational Radiology Laboratory. These are the first such investigations being done for these disorders. Promising results include a study, just submitted for publication, showing differences in the measurements of white-matter tracts in the brains of children with PMS versus typically-developing kids. Another study published in Pediatric Neurology last year showed decreased volumes of certain brain structures in the same population. We are conducting additional analysis of MRIs to better understand brain structure involvement in ASD.
- Comprehensive analyses of the three disorders’ neurobehavioral aspects, both individually and comparing across disorders. These have been submitted for publication, and we have made our data available to the scientific community. We welcome collaborations from all interested investigators.
- Development of genetic sample repositories. We have collected close to 2,000 blood samples from children with TSC, PMS, and PHTS, as well as their biological parents, siblings, and other family members. Over the next five years, we hope to complete detailed genetic analyses on these samples.
- Initiation of a pilot trial of everolimus in individuals aged 5 to 45 with a PTEN mutation, led by Antonio Hardan, MD, of Stanford University. This Phase I/II six-month, randomized, double-blind placebo-controlled trial is investigating safety and the effects of the drug on neurocognition. We have enrolled 34 participants to date at the three participating sites (Boston Children’s, Stanford, and Cleveland Clinic), and are on track to meet our goal of 40 enrolled by December 2019. Anecdotal results reported by parents are encouraging, and we expect the statistical analysis to be completed by December 2020.
- While the everolimus trial was a required pilot project of the grant, we took the initiative to conduct three additional pilots. One project, with financial support from the PMS Foundation, a patient advocacy group, aimed to study EEG biomarkers in children with PMS. The second pilot aimed to map the phenotype in adults with PMS. The third was a survey of patients and families of people with PHTS, investigating their access to resources, led by Siddharth Srivastava, MD, at Boston Children’s. Under his direction, Boston Children’s was able to develop a specialized PHTS clinic, one of only a handful around the world.
The next five years
Our new cycle of funding will allow us to continue our “deep phenotyping,” or detailed clinical characterizations of current and new participants. This will add to the rich data repository we have gathered for the three disorders. Extending our eligibility criteria to include adults up to age 45 will enable us to further understand disease trajectory and progression. This knowledge is especially important for those with PMS, who often appear to experience regression in language and adaptive functioning as they go into adulthood; some also develop psychiatric symptoms. Similarly, adulthood brings about new challenges for people with PHTS, especially new cancer occurrences that are not present in children. An important goal of our consortium is to understand these challenges and how treatment will be different for children versus adults.

We have also added an electrophysiological investigation that will inform us as we explore therapeutic strategies for these rare disorders. Led by Charles Nelson, PhD, director of the Laboratories of Cognitive Neuroscience, and Boston Children’s neurologist April Levin, MD, we aim to further identify EEG biomarkers of synaptic function and brain connectivity associated with ASD. While results in EEG and ASD research to date seem promising, we need further data to validate current findings in the field.
Finally, we will take steps to enable other investigators, inside and outside the DSC, to propose and implement projects and analyses as part of our Pilot Feasibility Core. This Core, led by Joseph Buxbaum, PhD, of the Seaver Autism Center and Mount Sinai School of Medicine in New York, will enable others to collaborate and use the infrastructure our consortium has put in place.

Beyond the science
In addition to scientific exploration, the consortium aims to support the research community and others involved in the treatment, care, support, and education of those affected by these disorders. That includes enhancing the careers and expertise of junior physician-scientists by training them in translational neurodevelopmental science through a two-year long Young Investigator Award, to be awarded in each disease group.
We also provide education to genetic counselors and students to enrich their knowledge about rare neurogenetic diseases. Most important to many clinicians and caregivers, we plan to develop consensus clinical care guidelines for both PMS and PHTS over the next couple of years, following a model that the TSC community created a few years back. Such guidelines will help define how best to manage clinical care of patients, setting a consistent global standard of care that can be covered by insurance agencies.

We are thankful for the support and activism of our patient advocacy partners: the TS Alliance, the PMS Foundation, the PTEN Hamartoma Tumor Syndrome Foundation, and PTEN Research, with which we have developed such great relationships. We are also grateful to the NIH for recognizing the value of rare disease research.
Related Posts :
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The hidden burden of solitude: How social withdrawal influences the adolescent brain
Adolescence is a period of social reorientation: a shift from a world centered on parents and family to one shaped ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...





