A journey through the intestine during colitis, cell by cell
Inflammatory bowel disease (IBD), causing devastating abdominal pain, persistent diarrhea, and rectal bleeding, is hard to control with current treatments. Children often experience malnutrition and impaired growth. To get a better handle on IBD, researchers at Boston Children’s Hospital and Brigham and Women’s Hospital decided to eavesdrop on happenings in the colon.
In the first effort of its kind, they mapped intestinal cells in mice — genetically, molecularly, and spatially — before, during, and after an episode of colitis. Jeffrey Moffitt, PhD, of Boston Children’s Program in Cellular and Molecular Medicine and Roni Nowarski, PhD, of Brigham and Women’s and the Blavatnik Institute, led the work with first authors Paolo Cadinu, PhD, in the Moffitt lab and Kisha Sivanathan, PhD, in the Nowarski lab.
The team used a molecular imaging method called MERFISH, co-developed by Moffitt, to image colon slices across multiple disease stages. They captured the expression of 940 genes in 1.3 million intestinal cells — producing an astronomical 50 terabytes of image data.
The findings, published in Cell, offer a new framework for understanding IBD and could spawn new types of interventions.
Diving into the intestine
Envision yourself inside a colon, surrounded by many different kinds of cells. Thanks to techniques like single-cell RNA sequencing, they’ve been catalogued into groups based on what genes are turned on and what signals they’re sending.
“Because our methods are very high throughput, we can look at millions of cells and discover populations that were missed before because they’re rare,” Moffitt says.
In all, Moffitt and his colleagues sorted the cells into 72 groups. They fell into eight major categories including: epithelial cells that line the intestinal wall, forming a protective barrier; fibroblasts, which lie beneath and help maintain the intestine; endothelial cells; cells of the enteric nervous system; smooth muscle cells; interstitial cells of Cajal, nerve cells responsible for intestinal contractions; and immune cells.
Now imagine that the cells are color-coded. Thanks to MERFISH, you can see where the different types reside and how they’re arranged in three-dimensional space.
“The gut is not just a bag of cells,” says Moffitt. “It’s a complex machine.”
“Other methods, like single-cell sequencing, give you the list of cell types — the parts,” elaborates Cadinu. “With MERFISH we can define and discover those parts while mapping how they fit together. This information is essential, as spatial organization shapes how cells interact with each other.”
In the midst of disease
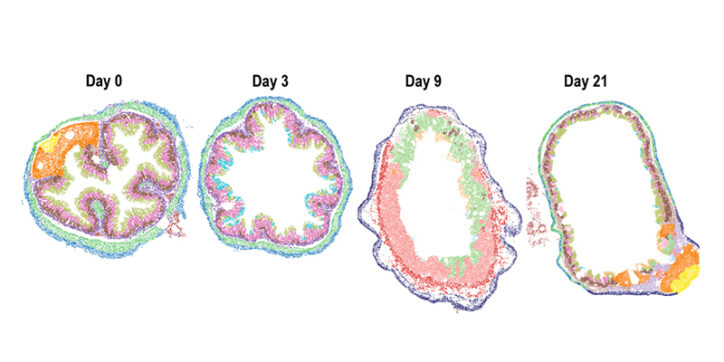
Now, imagine the intestine is having a flare-up of IBD. The inflammation begins to change it structurally and the cellular landscape shifts.
“When you induce colitis, you first activate small patches of the gut. You can see subtle changes in gene expression in the epithelial cell layer and in the fibroblasts right underneath,” says Moffitt. “Later, there are lots of structural changes.”
In the epithelial layer, the crypts — pockets where intestinal stem cells are made and maintain the intestinal lining — distort and start to flatten. Epithelial cells die off, and the barrier is breached. An ulcer forms, a sign of severe disease.
The intestine responds: The inflammation activates multiple types of fibroblasts. Through their action, immune cells flood into the ulcer, turn on antibacterial programs, and then are released into the intestinal cavity to defend against unwanted microbes.
Next up: Human studies
Fast forward two weeks, and the intestinal ulcers are starting to heal. Epithelial cells seal the barrier and the crypts start to repair themselves as intestinal stem cell numbers increase. At four weeks, regions of the intestine start to appear healthy. However, the fibroblasts that were activated by inflammation remain on alert.
“These long-lived activated fibroblasts might represent a form of tissue memory of the inflammatory insult, and could be important in later relapses of inflammation,” says Moffitt.
Moffitt thinks the work shows a new path for understanding many conditions, especially those involving inflammation. On the IBD front, the team plans to extend its methods to human clinical samples in collaboration with gastroenterology researchers at Boston Children’s.
“The mouse studies have highlighted exactly the features to investigate in human samples,” says Cadinu. “They highlight the importance of ulceration in disease, making this a priority for future studies.”
“The scale of measurements needed in humans with IBD is very challenging,” Moffitt acknowledges. “But we have to roll up our sleeves and rise to this challenge, with the hope that it will help patients have a better prognosis.”
Learn more about PCMM and research in the Division of Gastroenterology, Hepatology, and Nutrition
Related Posts :
-

Microvillus inclusion disease: From organoids to new treatments
Microvillus inclusion disease (MVID) is a rare type of congenital enteropathy in infants that causes devastating diarrhea and an inability ...
-

A new approach to C. diff? Targeting the inflammation, not the bacteria
Clostridium difficile (C. diff) intestinal infections can cause severe, debilitating diarrhea in patients who are hospitalized or on immunosuppressive therapies. ...
-

From bench to bedside: A promising option for unremitting ulcerative colitis
Many existing treatments for inflammatory bowel disease, like Remicade® and Humira®, work by blocking inflammatory cytokines such as tumor necrosis ...
-

Why do some people get severe COVID-19? The nose may know
The body’s first encounter with SARS-CoV-2, the virus behind COVID-19, happens in the nose and throat, or nasopharynx. A ...