Catching platelets with NETs: Neutrophils and deep vein thrombosis

Sea cucumbers have an unusual way of defending themselves. When threatened, they ensnare their foes with sticky threads. Some even expel their own internal organs to repel attackers.
Immune system cells called neutrophils sometimes do much the same: When confronted with bacteria, they unravel and shoot out their chromatin—the tightly wound mix of DNA and proteins that keeps genes packaged in cells. The resulting molecular mesh, known as a neutrophil extracellular trap, or NET, traps and kills bacteria, providing an additional line of defense against bloodstream infections.
But neutrophils and NETs can go awry. Since 2010, Denisa Wagner, PhD, of the Program in Cellular and Molecular Medicine at Boston Children’s Hospital, has been studying NETs’ role in deep vein thromboses (DVTs)—blood clots that form in veins deep in the body where blood clots shouldn’t, usually in the legs.
Unlike regular blood clots that stop bleeding from cuts and scrapes, DVTs form when a person is bedridden or sits still for a long period of time, like a long plane flight. If a DVT breaks loose, it can travel up through the bloodstream and get trapped in the lungs, where it can cause a potentially fatal pulmonary embolism.
If found early enough, it’s possible to treat DVTs with drugs like heparin, which interferes with blood clotting. But that kind of treatment can cause bleeds in other parts of the body.
This is where NETs come in. Three years ago, Wagner and one of her postdoctoral fellows, Tobias Fuchs, PhD, found that NETs can act like a scaffold for DVTs, catching, activating and amassing passing platelets. Later the Wagner lab showed that DNase, an enzyme that chews up DNA, could break DVT growth by digesting the NETs embedded in them.
But while Wagner’s prior work helps explain the ‘what’ of NETs and DVTs, it doesn’t explain the ‘why’ or ‘how.’ Why would neutrophils release NETs when there are no bacteria around? How do they do it? And is there a way to stop it?
Her latest study starts to answer those questions. Together with Pennsylvania State University’s Yanming Wang, PhD, Wagner and her graduate student, Kimberly Martinod, showed that an enzyme called PAD4 plays a part in weaving NETs.
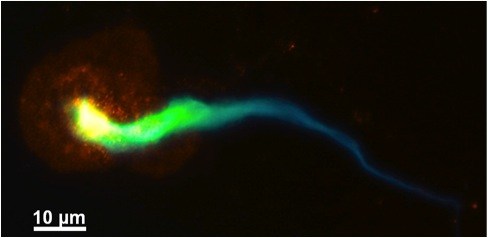
PAD4 gets neutrophils’ chromatin to start unraveling itself by changing its histones, the protein “pearl around which the DNA string is bound,” Wagner explains. Wagner, Martinod and Wang found that mice lacking the PAD4 gene couldn’t form NETs or DVTs anymore, yet could still form regular blood clots in response to injury. Transfusing those mice with neutrophils from mice that still had the gene brought DVT production back up to speed.
This understanding of NETs and how they’re produced, Wagner thinks, means there might be a better way to treat or prevent DVTs than traditional anticoagulants.
“It has long been thought that clot formation in DVTs relies solely on mechanisms that drive coagulation through thrombin and fibrin, which is why current treatments for DVTs focus on prevention or reversal of coagulation with agents like low molecular weight-heparin,” she recently said in a press release.
But enzymes like PAD4 are relatively easy to target for drug treatment, making Wagner optimistic that inhibiting PAD4 could help stop NET production in DVT cases. “We may have an opportunity here,” she says, “to create a new therapeutic approach for cases of DVT or pulmonary embolism, or one that targets both coagulation and neutrophils to provide better outcomes.”
Wagner is quick to note that while NETs were first discovered less than a decade ago, it’s clear that they have important roles up and down the evolutionary tree. “Plants wrap their root tips in structures like NETs to protect themselves from soil fungi, and some bacteria produce NET-like meshes within biofilms,” she says. “They’ve clearly been around for a long time, but we have only scratched the surface about what their functions might be, especially in us.”
Related Posts :
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

Bringing order to disorder: Jhullian Alston, PhD
Proteins typically fold into orderly, predictable three-dimensional structures that dictate how they will interact with other molecules. Jhulian Alston, PhD, ...
-

Tracking influenza in its first battleground: The nose
The answer to curbing influenza could be right under our noses — or, more accurately, inside them. New research maps happenings ...
-

A deeper understanding of inflammatory pain could reveal new solutions
Non-steroidal anti-inflammatory drugs (NSAIDs) such as ibuprofen are the main go-to for inflammatory pain caused by wounds, infection, sunburn, arthritis, ...