Could a GI bug’s toxin curb hard-to-treat breast cancer?
Clostridium difficile can cause devastating inflammatory gastrointestinal infections, with much of the damage inflicted by a toxin the bug produces. But research from Boston Children’s Hospital suggests that the same toxin could also be a useful tool for curbing highly aggressive triple-negative breast cancers that don’t respond to chemotherapy.
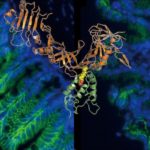
Min Dong, PhD, in the Department of Urology at Boston Children’s, is an expert on toxins produced by bacteria, including C. difficile’s toxin B. In 2016, his lab showed that toxin B gains entry into cells via a receptor known as Frizzled. Then, in 2018, the lab showed that when a fragment of toxin B binds to Frizzled, it prevents a molecule called Wnt from engaging with the Frizzled receptor — blocking Wnt signaling, which many cancers rely on for growth.
Could this property make this toxin fragment — which itself isn’t toxic — a useful cancer drug? Aine He, MD, PhD, a visiting oncologist in the Dong Lab, collaborated with the lab of cancer biologist Zhe Li, PhD, at Brigham and Women’s Hospital to test the concept in triple-negative breast cancer, also known as basal-like breast cancer.
A targeted effect
Frizzled is actually a family of receptors with 10 different members, all of which respond to Wnt signaling. However, previous studies had associated triple-negative breast cancer with specific Frizzled receptors. Dong, He, and colleagues further found that these cancer cells carry a specific subtype known as Frizzled 1/2/7. This prompted them to a toxin B fragment that targets Frizzled 1/2/7 but not other family members.
“Wnt signaling has been a promising target for cancer treatment,” says Dong. “But past efforts to develop effective inhibitors have often been hampered by side effects such as loss of bone density.”
Tested in several mouse models of basal-like breast cancer, the toxin fragment inhibited tumor-initiating cancer stem cells, chemotherapy-resistant mammary tumor cells, and growth of tumor organoids. Most notably, it inhibited the growth of tumors in mice without affecting bone or intestinal tissues, which also use Wnt signaling.
“Because toxin B is specific to the Frizzed 1/2/7 sub-family, it might be the narrow-spectrum inhibitor that we have been looking for,” says Dong. “It may offer an opportunity to inhibit tumor growth without the side effects.”
The findings appear in the November in PLoS Biology. The team has filed for a patent on the technology and hopes to develop an antibody mimicking toxin B for future clinical use.
For more information on this technology, contact Colm Lawler, PhD, in Boston Children’s Technology & Innovation Office.
Learn more about research in the Dong Lab.
Related Posts :
-

Science Seen: An intestinal toxin’s trick, a potential cancer fighter
Clostridium difficile, also called “C. diff,” causes severe gastrointestinal tract infections and tops the CDC’s list of urgent drug-resistant ...
-

Entry door for deadly C. difficile toxin suggests new mode of protection
Clostridium difficile, also called “C. diff,” tops the CDC’s list of urgent drug-resistant threats. Marked by severe ...
-

Stool transplant found safe, effective for 'C. diff' in children
Diarrhea caused by Clostridiodes (formerly Clostridium) difficile infections is on the rise among children; one population-based study found a 12.5-fold ...
-

Dually-targeted liposomes curb triple-negative breast cancer, metastases in mice
Some 15 to 20 percent of all breast cancers are triple-negative, meaning they lack receptors for estrogen, progesterone and human epidermal growth ...