Coordinated care and research for genetic cardiovascular disorders
Genetic cardiovascular disease in children sometimes comes to light in a crisis — a sudden collapse, sudden breathing difficulty, a sudden death in the family. Or it may be part of a diverse collection of symptoms, mostly having nothing to do with the heart. Sometimes it is picked up incidentally.
“From an incidental ECG finding when a child has their appendix out, you can unearth an inherited cardiac condition across an entire family,” says Dominic Abrams, MD, of the Cardiovascular Genetics Program at Boston Children’s Hospital.
In all, one half to one third of pediatric cardiology patients have a genetic etiology. Abrams has teamed up with cardiovascular geneticist Amy Roberts, MD, cardiac physician-scientists William Pu, MD, and Vassilios Bezzerides, MD, PhD, and cardiology subspecialists to create a comprehensive, family-based cardiovascular genetics program caring for children. It encompasses five main disease areas: arrhythmias, cardiomyopathies, congenital heart disease, aortopathies and related vascular diseases, and inherited lipid disorders.
The program unites genetics, cardiology, cardiac imaging, electrophysiology, psychology, social work, and genetic counseling — and, equally important, research and discovery.
“Our ultimate goal is to go full circle: evaluate a child, identify a genetic change, model their disease in the lab, and go back to the bedside with a treatment,” says Roberts.
Genetic cardiovascular disorders: A family affair
Often, a child’s diagnosis is part of a much larger family picture. The program’s ties to adult cardiology units in Boston — including a literal connection by bridge to Brigham and Women’s Hospital — allow family members to be cared for, evaluated, and genetically tested together, by the same team. Adult cardiologists who have seen a child’s parents can provide helpful input.
“It sounds very simple, but we don’t know of many other cohesive family-based programs,” says Abrams. “Families really appreciate it. I’ve had parents in tears, grateful that we were all in the same room working with the family.”
Cardiac care can even begin prenatally. Through the Maternal Fetal Care Center, teams of clinical specialists from Boston Children’s and Brigham and Women’s evaluate at-risk pregnancies. “Often, families who have had one child with a cardiac diagnosis ask about a second affected baby,” says Roberts. “Fetal echocardiograms, and, if indicated, prenatal genetic testing can help answer those questions.”

Ronald Lacro, MD; Steven Colan, MD; Renee Margossian, MD; Sarah De Ferranti, MD, MPH; Michael Singh, MD; Daniel Quiat, MD, PhD; and Elizabeth DeWitt, MD.
Genetics informing care
Now that genome and exome sequencing have come within clinical reach, genetic diagnostic capabilities have surged.
“In syndromic diseases that affect the heart, we’ve made leaps and bounds,” says Roberts. “We maintain registries of families who provide samples, so we can keep track of their genetic results and their medical and family history information as a tool for researchers.”
The registries help affected families find and support each other. They also help clinicians anticipate what to expect when a genetic diagnosis is made, guiding care.
For example, a study led by Abrams last year analyzed 32 children and young adults with arrhythmogenic cardiomyopathy (ACM). It showed that while people may not recognize symptoms until their 30s, signs can appear earlier in life. The study also connected the dots between anatomy, genetics, and symptoms: Right-ventricular ACM often manifested as rapid heart palpitations and fainting, while left-ventricular ACM often resembled myocarditis with severe chest pain. Each form was associated with different genes.
“One of the interesting questions we’re exploring is: What about people who have a genetic change and don’t have the condition?” says Abrams. “The ability to do exomes and genomes is helping us understand the prevalence of potentially disease-causing genetic mutations in the overall population.”
Teeing up treatments
Finding a mutation offers an opportunity to investigate cardiovascular biology — in the case of ACM, how the mutation affects proteins involved in electrical signaling and heart rhythms. Preclinical studies can then model the condition in a dish and in animals and test potential interventions.
One intervention drawing interest is gene therapy. Boston Children’s is on track to become a major center for cardiac gene therapy clinical trials. Its large, well-characterized patient cohorts are a draw for potential partners.
“Companies interested in translating new therapies to the clinic are attracted to Boston Children’s because of our unique combination of large, organized patient cohorts and integrated basic and clinical expertise,” says Pu, who directs the Basic and Translational Cardiovascular Research program.
In the past few years, Pu’s lab has laid the groundwork for gene therapy for single-gene conditions such as catecholaminergic polymorphic ventricular tachycardia (CPVT), a leading cause of sudden death from cardiac arrest. A multimillion-dollar grant, just awarded by the NIH’s Clinical Trials on a Chip program, will help move this work toward clinical trials.
Cardiac gene therapy
Last year, Pu and Kevin Kit Parker, PhD, of Boston Children’s and Harvard’s School of Engineering, Arts, and Sciences created the first human tissue model of CPVT, seeded with heart muscle cells carrying patients’ mutations (see image). They then ran an “exercise test in a dish,” using a drug to stress the muscle and infrared light to initiate faster heartbeats. This successfully replicated the patients’ arrhythmias and gave insights into their biology. Separately, a team led by Bezzerides used gene therapy to suppress CPVT arrhythmias in a mouse model.
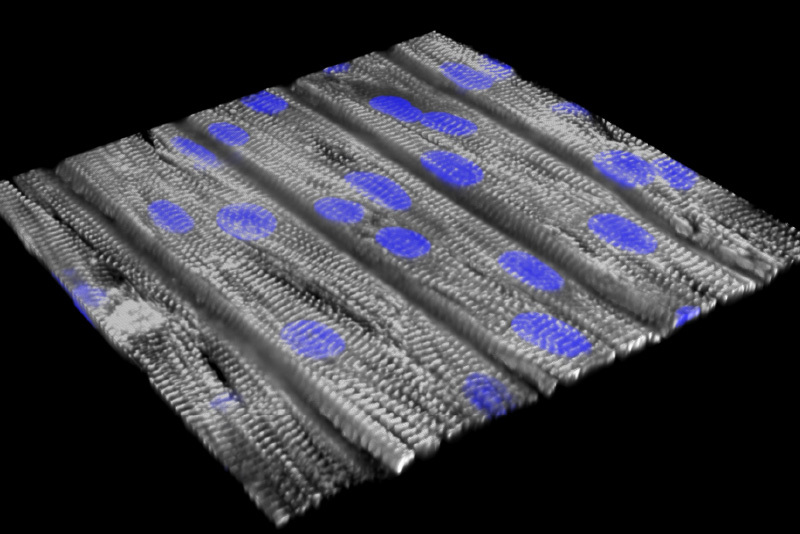
This work led to funding from the Massachusetts Life Sciences Center to create the Center for Accelerating Therapeutic Discovery, using use disease-on-a-chip models to facilitate translation of new therapeutics to the clinic. Pu and colleagues have also used gene therapy to correct Barth syndrome, another cause of life-threatening heart failure, using a mouse model that replicates both cardiac and skeletal weakness. They now are investigating ways to deliver the corrected gene more effectively to heart muscle.
Not all cardiovascular diseases lend themselves to gene therapy: many result from errors in multiple genes. But in theory, gene therapy could reverse any progressive heart disease with an identifiable genetic target. Pu and Bezzerides are now setting their sights on inherited heart diseases, including arrhythmogenic cardiomyopathy and hypertrophic cardiomyopathy, in which mutations cause the walls of the left ventricle to thicken and pump less effectively.
“There are still many unanswered questions about gene therapy,” notes Roberts. “No one knows how long a dose would last; there are challenges with delivery and off-target effects; and existing gene therapies are very expensive. We’re working actively on the research side to tackle these challenges.”
Learn more about the Cardiovascular Genetics Program at Boston Children’s.
Related Posts :
-

First-of-its-kind pressurization test could improve Ross procedure outcomes
The Ross procedure is a preferred surgery to treat severe aortic valve disease. The procedure replaces the failing valve ...
-

Promising advances in fetal therapy for vein of Galen malformation
In 2024, Megan Ingram* of California and her husband were preparing for the birth of their third child when a 34-week ...
-

Four things you should know about MAPCAs treatment
As the first grandchild in her family, Hannah Homan is in demand for frequent visits. She was also the focus ...
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...