Playing the long game: An exciting discovery in telomere disease

Each time our cells divide, the protective caps that keep our chromosomes from fraying, called telomeres, lose a bit of their DNA. Telomeres shorten steadily as we age, but in certain medical conditions like dyskeratosis congenita, the process is accelerated.
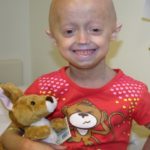
“Your telomeres determine your lifeline; how long they are determines how old your body is,” says Becca Hudson, who was diagnosed with dyskeratosis congenita at age 14. “My telomere length was below the first percentile for my age.”
A failing bone marrow
Trying out for cheerleading, 14-year-old Becca was pulled when testing found something amiss with her blood work. She had very low counts of platelets, red cells, and white cells. Her doctor called later that day and said she should be admitted that night to Boston Children’s Hospital.
Becca’s bone marrow was failing, unable to keep up with her need for healthy blood cells. She was first diagnosed with aplastic anemia. Later, a telomere length test led to the diagnosis of dyskeratosis congenita. Becca’s is one of about 300 cases known in the U.S.
Within months, in January 2015, Becca underwent a bone marrow transplant. She received an infusion of blood stem cells from a healthy donor through a protocol developed by her hematologist, Suneet Agarwal, MD, PhD, in the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.

While the transplant helped normalize Becca’s blood system, it didn’t slow down the shortening of telomeres in all her tissues. Although she has avoided serious complications of dyskeratosis congenita, such as liver disease or lung scarring, she suffers from a minor heart condition and joint pains.
“I describe it as an aging disorder,” says Becca, now 23 and just graduated from college. “My body thinks it’s older than it actually is. I’m grateful every day that I don’t have the worst of it.”
Extending telomere life
For more than a decade, Agarwal has looked for a way to lengthen telomeres and turn back the cellular aging process. Much of his lab’s work has focused on telomerase, an enzyme that builds telomeres back up, and how genetic mutations affect factors that make up telomerase or regulate it.
Now, taking an entirely different approach, Agarwal and Will Mannherz, an MD-PhD student in his lab, have zeroed in on a surprisingly effective way to lengthen telomeres — one that could be tested clinically relatively soon.
Using CRISPR editing, the researchers systematically knocked out genes in the genome one by one, looking for factors involved in telomere maintenance.
That revealed a surprising ingredient and potential therapeutic. As described recently in Nature Genetics, adding the compound thymidine lengthened telomeres dramatically in cell lines and in stem cells made from patients with dyskeratosis congenita.
Thymidine is one of four different nucleotides or “letters” of DNA, also known as bases, together with adenine, cytosine, and guanine.
“Nucleotides are what telomeres are made of,” says Mannherz. “We looked at all different combinations of nucleotides, and with every combination that had thymidine in it, telomeres were longer.”
Clinical trial planned
Serendipitously, thymidine has already been found safe and is being used in clinical trials for thymidine kinase 2 deficiency, another rare disease that weakens muscles. Agarwal, co-program leader for the Stem Cell Transplant Program at Boston Children’s, anticipates he could begin offering thymidine treatment to patients with telomere disease within a year or so. Eventually, he and his colleagues hope to establish a new treatment center focused on telomere diseases.
“We have many patients who are waiting for answers beyond bone marrow transplantation, which is only for the blood problems,” he says. “Because thymidine is already used and appears to be safe, we may save years compared to traditional drug development and de-risking.”
Becca, who has known Agarwal since her diagnosis, is excited by the prospect of trying thymidine. She’s long been involved in his research studies, donating blood and skin cells, and hopes to remain his patient as she transitions to adulthood.
“Sign me up!” she says. “If he does start a trial, I’m definitely going to try to jump in there.”
Learn about the Bone Marrow Failure and Myelodysplastic Syndrome Program at Boston Children’s.
Related Posts :
-

Not all heroes wear capes: Taking on dyskeratosis congenita
When Mason Langlais argues with his sister, Jillian, he regularly repeats a mantra. “I'm the rare one,” he says. “So, ...
-

First-ever drug trial reverses some signs of aging in progeria
The children came from all over the world: 28 families from 16 countries, speaking over a dozen languages. They faced a grim ...
-

A drug treatment for telomere diseases?
For years, Donna Martin carried a piece of scrap paper with the words “dyskeratosis congenita,” which she believed might explain ...
-

Chromosomal testing expands options for exploring causes of SIDS
When an infant or young child dies without explanation, it is not uncommon for parents to blame themselves. In some ...