Genetic analysis backs a neuroimmune view of schizophrenia: Complement gone amok
A deep genetic analysis, involving nearly 65,000 people, finds a surprising risk factor for schizophrenia: variation in an immune molecule best known for its role in containing infection, known as complement component 4 or C4.
The findings, published this week in Nature, also support the emerging idea that schizophrenia is a disease of synaptic pruning, and could lead to much-needed new approaches to this elusive, devastating illness.
The genetic tour-de-force was pulled off by the Broad Institute and Harvard Medical School (HMS), in collaboration with the Boston Children’s Hospital lab of Beth Stevens, PhD, and the lab of Michael Carroll, PhD, of HMS and the Program in Cellular and Molecular Medicine at Boston Children’s.
While pruning connections between neurons is part of normal brain development — a way for the brain to shed irrelevant or dysfunctional synapses — the new research suggests that too much or too-prolonged pruning can lead to schizophrenia as people enter their teens or young adulthood. This jibes with the observation that people with schizophrenia tend to have thinner cortical tissue, reflecting a loss of synapses.
A strong immune signal
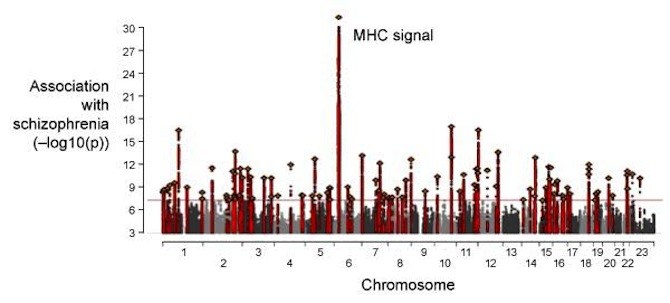
The Broad Institute researchers, led by Steven McCarroll, PhD, director of genetics for the Stanley Center for Psychiatric Research and associate professor of genetics at HMS, began with prior data from large-scale, international genome-wide association studies (GWAS) that Broad had helped lead. These studies pointed to more than 100 regions of the genome with genetic variations associated with schizophrenia.
One hit was especially strong and intriguing: a region known as the major histocompatibility complex (MHC) locus. It contained hundreds of genes with roles in the immune system.
This was a pretty big target. The Broad team, however, noticed clues pointing to immune factors known as complement components — a group of molecules that mark pathogens for destruction by immune cells. Stevens, a recent recipient of the MacArthur “genius” grant, had led studies at Boston Children’s F.M. Kirby Neurobiology Center showing that complement molecules also flag synapses for elimination.
Signs seemed to point in particular to genes for complement component 4 (C4), which became the group’s focus. C4 genes have a complex form of variation that wasn’t fully understood; people appeared to have widely different numbers of copies of these genes, as well as different forms (C4A and C4B).
“These have been challenging problems to solve because they require new combinations of genetics, biology and math,” says McCarroll.
Establishing the genetic link
Developing his own molecular methods, Aswin Sekar, an MD/PhD student in McCarroll’s lab, found more than a dozen structural forms of C4. Studying postmortem brain samples from schizophrenic patients, he and McCarroll showed that patients with particular structural forms of the C4 gene had greater C4 activity in their brains.
Sekar then figured out a way to correlate his data with huge datasets that tracked schizophrenia against SNPs — straightforward, single-letter DNA changes. This data crunch confirmed that patients with certain forms of C4 indeed had a higher likelihood of schizophrenia, and that the C4 gene was more active in brain tissue from people with schizophrenia compared with unaffected people.
But what was the underlying biology? For this, they turned to Stevens, who was happy to collaborate.
Salvaging synapses
“When they came to me with this result, it was amazing, it was exciting,” Stevens says in this Broad video. “They knew my lab had been studying complement and its role in normal pruning.”
Stevens and colleagues’ research had previously implicated another complement component, C3, in tagging synapses for pruning, cuing other immune cells known as microglia to come in and dispatch them. New experiments in her lab showed that the C4 protein is also present in human neurons and that it, too, localizes to synapses.
Michael Carroll, PhD, at Boston Children’s/Harvard then joined the effort. He had developed mice with different numbers of copies of C4. Together, the three research groups showed that C4 was necessary for C3 to be deposited onto synapses. Mice lacking the C4 gene had reduced levels of synaptic pruning. The data also suggested that the more C4 activity an animal had, the more synapses were eliminated in its brain at a key time in development.
The scientists now plan to develop mouse models based on human C4 variants to explore the role of C4 in pruning in the human brain and whether it is part of other neurological disorders.
While the C4 work doesn’t have immediate therapeutic implications for schizophrenia, the potential is there to address schizophrenia at its roots by dialing down pruning, rather than trying to treat it symptom by symptom. Drugs that inhibit other complement factors related to C4 are already under development for other diseases.
“This study marks a crucial turning point in the fight against mental illness,” said Bruce Cuthbert, PhD, acting director of the National Institute of Mental Health, in a Broad press release. “Because the molecular origins of psychiatric diseases are little understood, efforts by pharmaceutical companies to pursue new therapeutics are few and far between. This study changes the game. Thanks to this genetic breakthrough we can finally see the potential for clinical tests, early detection, new treatments and even prevention.”
Explore the findings in depth in Broad’s press release and news story, learn more about Beth Stevens’s work and read coverage in the New York Times and Washington Post.
Related Posts :
-

The hidden burden of solitude: How social withdrawal influences the adolescent brain
Adolescence is a period of social reorientation: a shift from a world centered on parents and family to one shaped ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

New research shows caregiver instability affects development
According to some estimates, more than 100 million children around the world experience separations from their caregiver every year. Previous research — ...
-

Study highlights the severity of acute necrotizing encephalopathy in kids with the flu
For most children, influenza (flu) usually means unpleasant symptoms like a fever, sore throat, and achy muscles. But for a ...