Remdesivir: What to know about the first drug approved to treat COVID-19

In late October, the U.S. Food and Drug Administration (FDA) officially approved the antiviral drug remdesivir (Veklury) for COVID-19. The approval has been controversial, with many scientists questioning the strength of the evidence and proposing that the drug undergo further clinical trials before receiving full FDA approval .
What does this mean for children who test positive for the coronavirus? Most otherwise healthy children with COVID-19 have very mild illness. As a result, they would be unlikely to receive remdesivir or other medications given their likely rapid recovery.
The FDA’s approval of remdesivir is for adults and children 12 years and older who are sick enough to be in the hospital. However, most children under 12 can still receive remdesivir if they are hospitalized. The drug is available either through ongoing clinical trials or under FDA Emergency Use Authorization (which allows the use of unapproved drugs in severe or life-threatening situations).
For more, we sat down with Dr. Karen Ocwieja, a physician scientist in Boston Children’s Hospital’s Division of Infectious Diseases.
What do we know about remdesivir?
The FDA based its approval of remdesivir on three randomized trials in adults with mild to severe COVID-19.
How remdesivir works
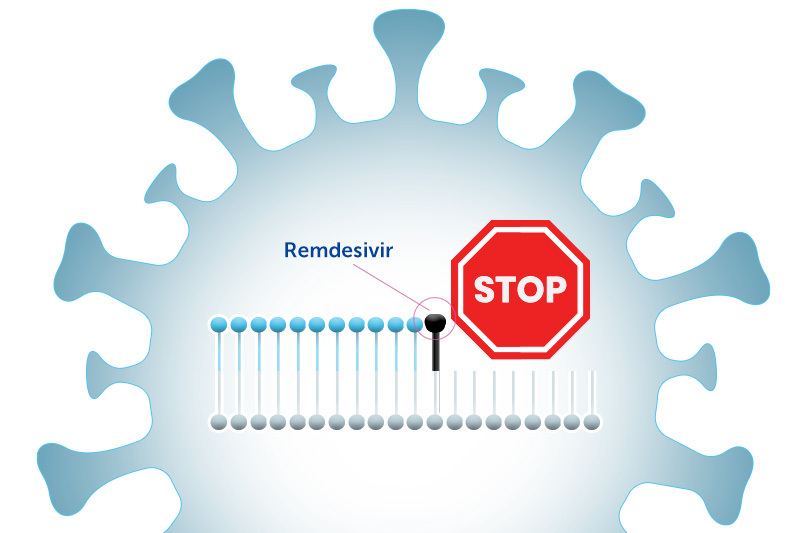
In order to make copies of itself, the coronavirus has to copy its genome, which is made up of building blocks called nucleosides that are linked together like Legos. Remdesivir blocks this process. It “looks” like a nucleoside, so the virus inserts it into its genome. This stops the genome copying process in its tracks, and prevents the virus from replicating.
The largest and most rigorous trial, published October 8, 2020 in The New England Journal of Medicine, involved about 1,000 adults. It showed a shorter time to recovery with remdesivir: 10 days, versus 15 days with a placebo. The investigators noted no overall improvement in survival. However, patients who required oxygen, but not mechanical ventilation at the start had a slightly higher survival rate.
The other two trials, both primarily in adults, offered weaker evidence of benefit. One, published in The New England Journal of Medicine in May, found clinical improvement in patients with severe disease. But there was no placebo group for comparison, making it hard to assess whether the improvement was actually due to the drug. Another study, published in JAMA in September, compared remdesivir with standard care. At 28 days, there was no difference in mortality or time to recovery.
In all three trials, remdesivir appeared to be safe. There was also a much larger study sponsored by the World Health Organization (WHO). Its interim results, published October 15, have not yet been fully reviewed by independent scientists. However, they showed no significant difference in mortality, length of hospitalization, or need for a ventilator.
Does remdesivir work in children? Is it safe?
Currently, data related to the use of remdesivir in children are limited. Of the three trials on which the FDA based its approval, two included a small number of children ages 12 and older. Gilead Sciences is currently sponsoring an international clinical trial in children. Boston Children’s is part of that scientific study, which may provide more answers.
In July, Gilead released preliminary information on 77 children with COVID-19 who received remdesivir through the company’s compassionate use program. The drug appeared safe, and 83 percent of the children had recovered from COVID-19 by day 28. But there was no comparison group, so we don’t know how these children would have done without remdesivir.
Is remdesivir used for COVID-19 at Boston Children’s?
We don’t yet know how much remdesivir may help children with COVID-19, and we would like to continue collecting data on its efficacy. But based on the safety data, the risks of remdesivir appear to be low for most patients. Boston Children’s is currently prescribing remdesivir in children with severe COVID-19 symptoms and those who require intensive care. The drug is available through either clinical trials, like Gilead’s trial, or Emergency Use protocols.
We continue to monitor data as it becomes available to help determine who might benefit. Remdesivir has shown a range of side effects, including gastrointestinal upset and mild, reversible liver toxicities, so we monitor children closely when we use it.
While remdesivir is the first drug approved by the FDA to treat COVID-19, many more drugs are being tested, along with a number of different vaccines. Time will tell what preventive and treatment measures are most beneficial in fighting this virus.
Read more about Boston Children’s response to COVID-19.
Related Posts :
-

Creating the next generation of mRNA vaccines
During the COVID-19 pandemic, mRNA vaccines came to the rescue, developed in record time and saving lives worldwide. Researchers in ...
-

The gut-brain connection: A new approach to OCD and tic disorders?
It’s natural for young children to use routines to help them navigate the world and for older children and ...
-

Can we prevent seizures in Sturge-Weber syndrome?
Port wine stains — capillary malformations on the skin — are the most visible manifestation of Sturge-Weber syndrome. However, up to 60 percent ...
-

Helping Jasmine manage Sturge-Weber syndrome before symptoms start
Sturge-Weber syndrome is a rare neurovascular disorder that increases the risk of seizures in infants due to abnormal blood vessel ...