Study suggests hypoxia overexpression causes pericytes to contribute to pulmonary hypertension
Pericytes, the multifunctional cells that work within the walls of capillaries, have been a subject of focus in the study of vascular development, cerebral blood flow, cancer, and neurodevelopment diseases.
But pericytes hadn’t been truly studied for their potential role in pulmonary arterial hypertension (PAH) until they landed under the microscopes of Boston Children’s researchers. They recently discovered that pericytes appear to contribute to the development of PAH.
The overexpression of hypoxia in pericytes contributed to severe pulmonary hypertension (PH) and right ventricular hypertrophy, whereas their removal of hypoxia relieved PH, according to Ke Yuan, PhD, an associate scientific researcher in Boston Children’s Division of Respiratory Diseases Research who was a co-corresponding author of the study. Her team hopes their discovery can eventually lead to treatment.
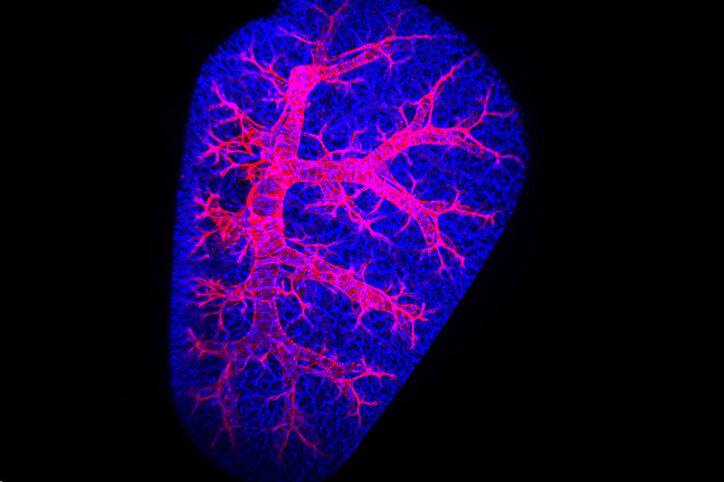
Focusing on pericytes in vascular remodeling
One reason why researchers hadn’t focused on pericytes is they’re difficult to get into focus. The tiny size of capillaries (they’re no more than 10 micrometers in diameter) hasn’t helped. A lung’s structure can also collapse during a study, preventing researchers from having a 3D perspective that can show the whole context of pericytes’ function. And pericytes share genetic markers with the other mural cell of vasculature — the smooth muscle cell — making them hard to distinguish from one another.
Yuan’s lab solved those problems by using confocal microscopy and light sheet microscopy to get an in-depth look at capillaries in super high resolution and see the full context of pericyte behavior while studying the lungs of mice and human PAH samples.
Their study, which was published in EMBO reports, comes as respiratory researchers learn more about pericytes as well as the vascular remodeling that triggers pulmonary hypertension (PH). One such finding was that pericytes may change into vascular muscle cells under stress or injury. Other studies showed how lung blood vessels exposed to hypoxia were blocked by a proliferation of smooth muscle cells. Yuan’s lab wanted to learn if hypoxia similarly led pericytes to undertake the kind of vascular remodeling that would lead to PAH. “We were interested in seeing how pericytes move from a capillary to an artery, that initiation step,” she says.
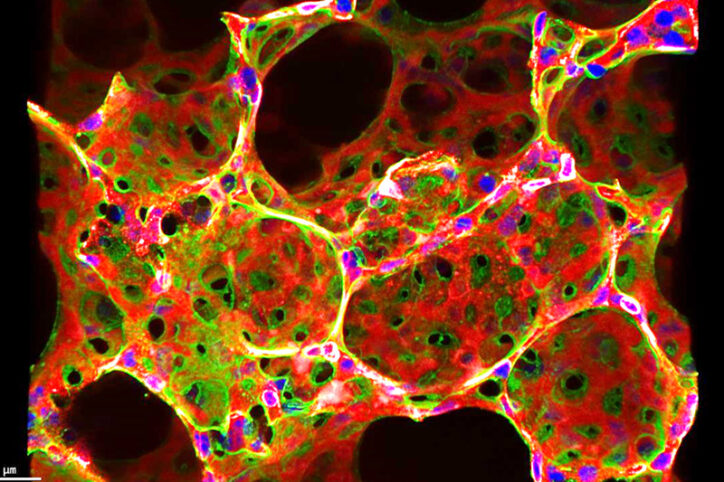
Treatment could target hypoxia signaling
Using single-cell RNA-sequenced and hypoxia-induced PH models, Yuan’s lab found that hypoxia-inducible factor-2a (HIF-2a) signaling plays an important role in pericytes’ transformation into smooth muscle-like cells in PH. Tracing pericytes’ cellular lineage, Yuan’s lab saw that they relocated from capillaries to arterioles and co-expressed smooth muscle actin. They also found that HIF-2a overexpression in pericyte-dominant transgenic mice exacerbates PH and right ventricular hypertrophy.
Their finding could open a door to treatment that targets HIF-2a signaling in pericytes. “If we find out how pericytes change lineage and are associated with pulmonary hypertension, we want to design therapies to prevent them from becoming smooth muscle-like cells,” Yuan says.
Until then, Yuan and colleagues need to learn more about pericytes and PAH. They hope to better distinguish pericytes from smooth muscle cells and other minor cells in capillaries so that tools can be developed to improve lineage tracing studies. Finding out how pericytes contribute to right ventricular hypertrophy is also a goal.
“Pericytes are abundant all over the body, but they’ve never been studied for their role in pulmonary or cardiovascular diseases,” she says. “They’ve been overlooked, but our work suggests they contribute to the vascular remodeling process behind pulmonary hypertension and other cardiovascular diseases.”
Read how the Pulmonary Hypertension Program treats children.
Related Posts :
-

Parsing the promise of inosine for neurogenic bladder
Spinal cord damage — whether from traumatic injury or conditions such as spina bifida — can have a profound impact on bladder ...
-

Unveiling the hidden impact of moyamoya disease: Brain injury without symptoms
Moyamoya disease — a rare, progressive condition that narrows the brain’s blood vessels — leads to an increased risk of stroke ...
-

Forecasting the future for childhood cancer survivors
Children are much more likely to survive cancer today than 50 years ago. Unfortunately, as adults, many of them develop cardiovascular ...
-

Genomic sequencing transforms a life: Asa’s story
Asa Cibelli feels like he’s been reborn. The straight-A middle schooler plays basketball and football, does jiu jitsu, is ...