Shoring up heart muscle’s mini ‘managers’ to treat heart failure
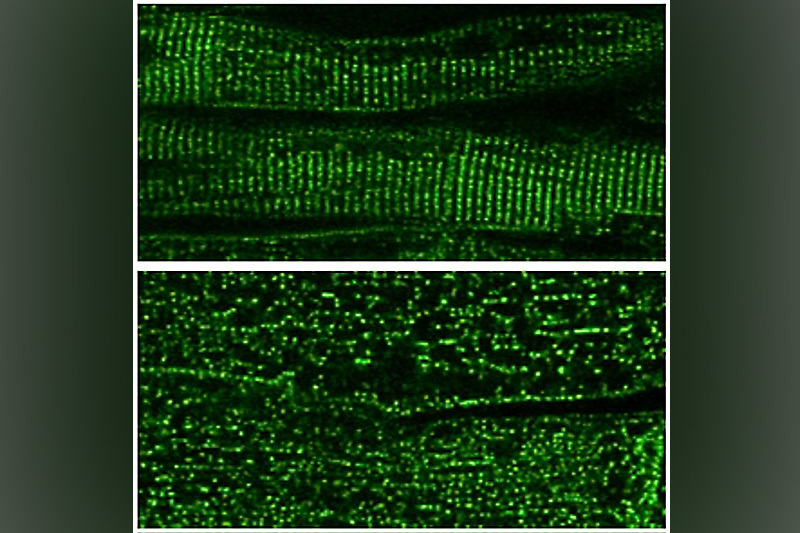
Our heart muscle is studded with tiny dyads, intricately designed structures that manage incoming electrical signals and calcium release to coordinate our heartbeats. Could gene therapy help maintain dyads’ structure and boost the function of failing hearts? A new study suggests it can.
“We know that in heart failure from many causes, dyads become disorganized,” says study leader William Pu, MD, director of Basic and Translational Cardiovascular Research at Boston Children’s Hospital. “Dyad disorganization is not usually an originator of heart failure, but can worsen it. Having a way to preserve dyad organization could be an approach to treating many different types of heart failure.”
In previous work, Pu and colleagues showed that a protein called cardiomyopathy-associated 5 (CMYA5) is important for assembling and stabilizing dyads. Now, they’ve figured out a way to deliver extra CMYA5 to heart muscle cells, or cardiomyocytes, through gene therapy. The promising early results appeared recently in Nature Biomedical Engineering.
CMYA5 gene therapy improves dyad structure, heart function
The team first whittled down the CMYA5 gene to those portions that appear most important to its function.
“The CMYA5 protein is enormous, much too large to put into a gene therapy vector,” Pu explains. “So we had to engineer the protein to make it small enough to fit.”
The researchers then conducted several tests, starting with a mouse model in which the CMYA5 gene was knocked out. The mice showed disorganization of their dyads and had weakened heart function. But both problems were reversed when they received gene therapy, delivered by a non-infectious adeno-associated virus.
The team then turned to genetically normal mice that had high-pressure loads on their hearts. This cardiac stress led to disorganized dyads and simulated the effects of medical conditions that lead to heart failure, such as stenosis or narrowing of the aortic valve, hypertension, and coarctation of the aorta. Mice that received mini-CMYA5 gene therapy had improved dyad structure, improved heart function, and reduced cardiac hypertrophy on echocardiograms and did not develop heart failure. The gene therapy improved myocardial outcomes even when cardiac dysfunction was already established.
Other experiments looked at the effects of mini-CMYA5 gene therapy on human cardiomyocytes derived from stem cells. These cardiomyocytes typically don’t make dyads, perhaps because they are not fully mature, notes Pu.
The miniatured CMYA5 helped the cardiomyocytes make some of the structures of dyads, helped organize the dyads’ architecture and coordination of calcium release, and increased the cells’ contractile force.
Applications in arrhythmia and myopathies?
Pu notes that CMYA5 gene therapy could potentially improve arrhythmias by helping coordinate calcium release. It might also be improve myopathies in skeletal muscle, which also has high levels of CMYA5.
His team, for now, is focused on the basics: understanding why there is less CMYA5 protein in heart failure, how this contributes to dyad disorganization, and how to optimize mini-CMYA5 to make it more powerful.
“Mini-CMYA5 protected against heart failure in the models we used, but the protection was incomplete,” Pu says. “There’s room for improvement.”
Explore clinical studies at the Heart Center and subscribe to Boston Children’s monthly research newsletter.
Related Posts :
-

Getting to the heart of heart muscle function
Every heart muscle cell, or cardiomyocyte, is studded with tiny, intricate structures called dyads. The dyads are like orchestra conductors: ...
-

Finding a possible genetic treatment for rare arrhythmias
Variants in a gene that plays a key role in heart function can cause potentially life-threatening arrhythmia syndromes known as ...
-

In the genetics of congenital heart disease, noncoding DNA fills in some blanks
Researchers have been chipping away at the genetic causes of congenital heart disease (CHD) for a couple of decades. About 45 ...
-

Coordinated care and research for genetic cardiovascular disorders
Genetic cardiovascular disease in children sometimes comes to light in a crisis — a sudden collapse, sudden breathing difficulty, a sudden ...