How do patients with cystic fibrosis respond to COVID-19? An ‘airway in a dish’ may give answers
So far, based on clinical data, patients with cystic fibrosis (CF) don’t appear to be especially susceptible to COVID-19. And when they do get infected, they don’t seem to get sicker. But Ruobing (Ruby) Wang, MD, who cares for patients with CF in the Division of Pulmonary Medicine at Boston Children’s Hospital, thinks there is more to the story.
“We know that when patients with CF got H1N1, some got very sick,” she says. “Because the CF community has been so effective at social distancing, there is a lack of information on how they respond to SARS-CoV-2.”
Based on the experience with H1N1 and other infections, Wang suspects that patients with CF may have different susceptibility to COVID-19, a dysregulated immune response, or both. Under a grant from the Cystic Fibrosis Foundation, she is putting these questions to the test.
Using patients’ blood cells, stem cell technology, and gene-editing technology, Wang and her colleagues at Boston University have engineered an airway lining that reproduces CF in a dish. They are now testing what happens when they expose this model to SARS-CoV-2. They are also conducting tests in a comparison model, in which they corrected the CF defect through CRISPR-Cas9 gene editing.
“We did this to create a clean experiment,” explains Wang. “We corrected the CF mutation, but otherwise, the cells come from the same patient with the same genetic background. This ensures that any observed difference in response to the virus is due to the CF defect.”
Building a CF airway
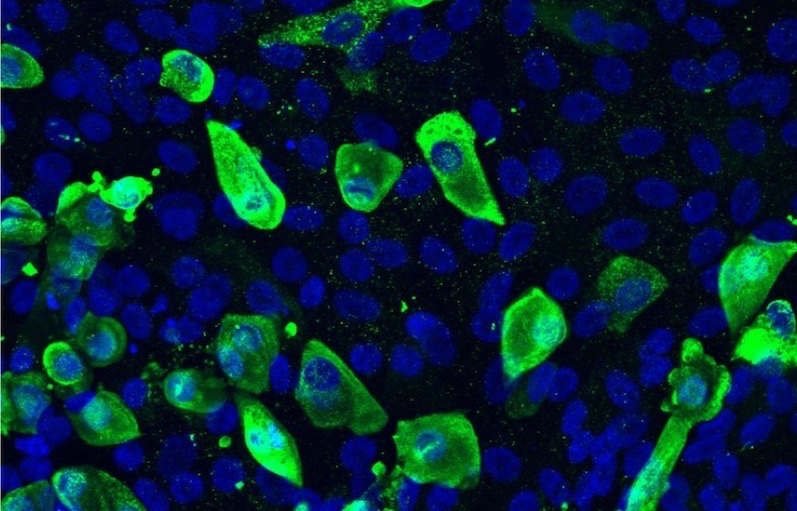
Wang’s team built on an airway model developed last year by her collaborators, including Darrell Kotton, MD, and Finn Hawkins, MB, BCh, in BU’s Center for Regenerative Medicine. Created from induced pluripotent stem cells (iPS cells) made from blood cells, the model contains all the major airway cell types, including stem-like airway basal cells, ciliated cells, and secretory cells.
For the new model, Wang generated the airways using stem cells derived from some of her own patients with CF. In experiments, the model reproduced CF’s hallmark defect in ion transport. But when her team corrected the CFTR gene in the stem cells — aided by Thorsten Schlaeger, PhD, and George Daley, MD, PhD, in Boston Children’s Stem Cell Program — the airway cells made from the corrected cells were free of the defect.
“This provided us with perfectly matched control samples to investigate the role of CFTR in health and disease, including responses to COVID-19 infection,” says Wang.
A coronavirus challenge
As the next step, Wang’s team, with Hawkins and Kotton, has begun exposing its CF airway model to live SARS-CoV-2. For safety purposes, they are doing this work at the National Emerging Infectious Disease Laboratories Biosafety Level 3 facility at BU, with Elke Muhlberger, PhD, Mohsan Saeed, PhD, and others.
Trial tests dornase alfa for COVID pneumonia
“To our knowledge, this is the first time an airway derived from iPS cells is being used to model the effects of SARS-CoV-2,” notes Wang.
Thus far, the researchers have shown that the model CF airway can be infected by SARS-CoV-2 and can be potentially used to assess antiviral drug responses. They believe this airway-in-a-dish could be a stand-in for modeling the effects of SARS-CoV-2 in patients with CF and testing a patient’s personalized response to treatments. In future research, Wang hopes to compare responses to the virus — and to antiviral drugs — in model CF airways versus genetically corrected, “non-CF” airways.
“Information from these studies may generate useful insights to guide therapy for CF patients during the COVID-19 pandemic,” Wang says.
Down the road, her team could use the platform to test the airway effects of other viruses, toxins, or diseases. “I’d love to see how an asthmatic airway responds to the coronavirus too,” she says.
Learn more about the Boston Children’s Division of Pulmonary Medicine.
Related Posts :
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

Modeling urinary tract disorders on a chip: Zohreh Izadifar
When a new tissue sample arrives from the Department of Urology, the Boston Children’s Hospital lab of Zohreh Izadifar, ...
-

Mapping cells to create targeted treatments for interstitial lung disease
John Kennedy, MD, MSc, remembers the relative simplicity of his first genetic mapping project. In a Harvard Medical School&...
-

Blood across our lifetimes: An age-specific ‘atlas’ tells a dynamic story
The stem cells that form our blood, also known as hematopoietic stem cells (HSCs), are with us throughout our lives. ...





