2020, the year COVID-19 tuned us into science: Part 3
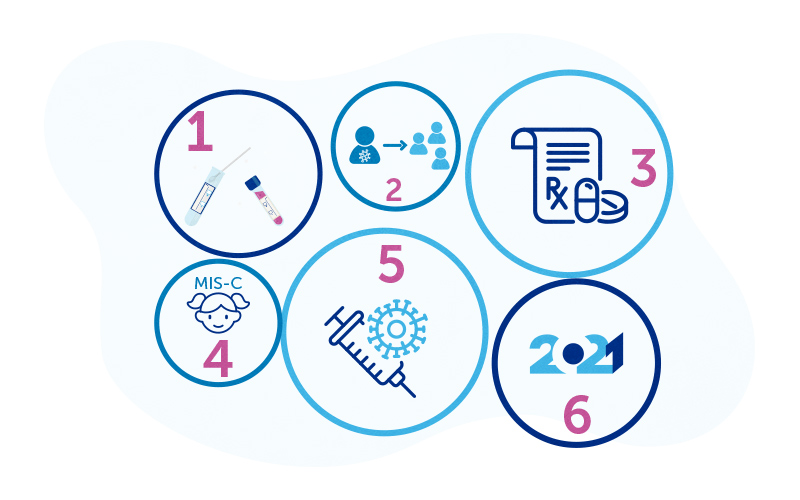
Since the arrival of a new, unknown, dangerous virus in January, we entered the realm of COVID-19 science. Part 1 and Part 2 of this series recapped what we learned about how the virus spreads, how to test for it and treat it, and how COVID-19 plays out in children. This month, vaccines began to be available — not a moment too soon, but much sooner than expected. But there is still so much to learn — about the vaccines and COVID-19 in general.
Lesson #5: Rapid development of coronavirus vaccines
In November, we received encouraging news: three potential COVID-19 vaccines (from AstraZeneca, Moderna, and Pfizer) completed Phase 3 trials, showing efficacy of up to 95 percent when tested against a placebo in large numbers of adult patients. The FDA approved both vaccines this month, and immunizations have finally begun, mainly in health care workers. So far, aside from a small number of allergic reactions, no major safety concerns have been reported.
But hurdles lie ahead. The three vaccines above each require two doses, and some vaccines require extreme sub-zero storage temperatures. We don’t yet know how long protection will last, or what safety problems might surface as millions of people get vaccinated. Scientists continue to learn about how the immune system reacts to SARS-CoV-2, including runaway immune reactions in some people known as “cytokine storms.” We need to know that the vaccines are safe and don’t trigger these harmful responses. Finally, testing in children is only beginning, and we may need to wait for some time before approval for vaccine use in children.
Lesson #6: What we still don’t know: Looking to 2021
As much as we’ve learned about COVID-19 in 2020, there’s even more we don’t know. We don’t yet understand why preexisting conditions like diabetes or kidney disease put people at higher risk. Or why some of us with no apparent risk factors get so sick with COVID-19 and MIS-C. Boston Children’s Hospital is playing a leading role in two large national studies looking closely at how the immune system reacts to SARS-CoV-2 and how children in particular handle the virus. Another study is looking for genetic changes that might make some children vulnerable to MIS-C and what might protect them.
Assuming the new vaccines are effective, will enough people accept them that we attain herd immunity — enough to protect the whole population? Will an increased understanding of the virus lead to treatments that make COVID-19 manageable? Will we have accurate tests that we can rely on? In the coming year, we’ll continue to watch the science unfold.
Read the rest of the series:
Part 1: How COVID-19 spreads and the science of staying safe; COVID-19 tests
Part 2: COVID-19 treatments; COVID in children and the emergence of MIS-C
Learn more about Boston Children’s response to COVID-19 and more about COVID-19 research at Boston Children’s.
Related Posts :
-

BRD7 research points to alternative insulin signaling pathway
Bromodomain-containing protein 7 (BRD7) was initially identified as a tumor suppressor, but further research has shown it has a broader role ...
-

When diagnosis is just the first step: The Brain Gene Registry
Through advances in genetic sequencing, many children with rare, unidentified neurodevelopmental disorders are finally having their mysteries solved. But are ...
-

Engineered cartilage could turn the tide for patients with osteoarthritis
About one in seven adults live with degenerative joint disease, also known as osteoarthritis (OA). In recent years, as anterior ...
-

Surgery beats sclerotherapy for rectal prolapse in children ages 5 and older
Rectal prolapse — the protrusion of the lining of a child’s rectum through the anal sphincter — can occur for many ...