Preventing leukemia by preventing rogue blood cells from taking over
As we age, many of us acquire mutations that cause some of our blood stem cells to multiply faster than others, forming their own distinct populations or “clones.” This is known as clonal hematopoiesis. In some cases, a single clone originating from a single genetically altered or mutated stem cell can expand to comprise up to 30 percent of a person’s blood cells.
If such a “rogue” clone acquires more mutations, it can lead to myelodysplasia, a rare blood disease, and in turn to leukemia. The lab of Leonard Zon, MD, director of the Stem Cell Research program at Boston Children’s Hospital, wondered if these rogue populations could be nipped in the bud, preventing a potentially fatal leukemia from ever starting.
If you could understand how clones of blood stem cells become cancerous, you could target the offending clone that’s causing the problem.”
If so, it could help both adults who develop clonal hematopoiesis as they age and children with a variety of blood disorders like Shwachman-Diamond syndrome, GATA2 deficiency, or RUNX1 familial platelet disorder, in which clonal hematopoiesis can develop in childhood.
“These children have a germline mutation that puts them at risk for developing leukemia earlier in life,” explains Serine Avagyan, MD, PhD, a pediatric oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center and a fellow in the Zon lab. “They can develop clonal hematopoiesis in adolescence and sometimes at a younger age. We think these predisposition syndromes fast-forward the process of clonal hematopoiesis, resulting in an early-onset leukemia.”
Tracking blood stem cells with colored ‘barcodes’
Certain stem cell populations likely come to dominate — and ultimately become cancerous — because they gain some kind of edge through a genetic mutation. Or, as Charles Darwin might have put it, they become more “fit.” But how?
“If you could understand how clones of blood stem cells become cancerous, you could specifically target the offending clone that’s causing the problem,” says Zon.
That’s where Zon’s team, led by Avagyan and Jonathan Henninger, PhD, now at the Whitehead Institute for Biomedical Research, turned to zebrafish. A favorite tool of the Zon Lab, zebrafish are easy to breed and their embryos are translucent. That see-through quality enables scientists to watch developing blood cells in real time and observe the effects of different genetic mutations.

Once an errant gene is found, scientists can test multiple drugs at a mass scale, simply by adding them to the fishes’ water. To date, Zon credits zebrafish for revealing four different drugs that have gone into clinical trials for melanoma, Diamond-Blackfan anemia, and adenoid cystic carcinoma, as well as a drug to enhance cord blood transplants.
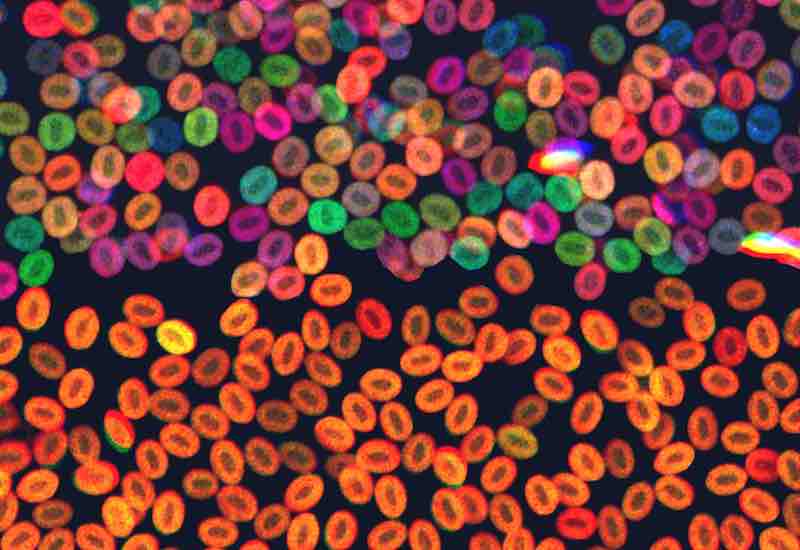
In this case, Zon’s team used a zebrafish model called Zebrabow in which different colors serve as “barcodes” of a cell’s identity. In normal zebrafish, blood stem cells are of multiple colors, indicating their diversity. Using CRISPR, the team introduced different mutations found in human clonal hematopoiesis into the embryonic fish. They then watched to see whether one color of stem cell became dominant — indicating that a rogue population of blood cells had taken hold.
“Serine was able to create mosaic mutations, such that some of the blood stem cells had the mutation and others didn’t,” says Zon. “We could then ‘compete’ the cells in the fish’s body to see which mutations caused a certain line of cells to take over.”
Suppressing rogue stem cells to curb leukemia
Indeed, certain mutations, such as in the gene asxl1, did lead to one blood stem cell population — and a single color of cell — becoming dominant in the zebrafish. The researchers then went further, examining what genes different kinds of blood cells were turning on as a result.
Mature white blood cells with mutations that cause clonal hematopoiesis turned on a host of inflammatory genes. In contrast, mutated blood stem cells turned on anti-inflammatory genes and were making anti-inflammatory molecules — protecting themselves against inflammation. When the team knocked out one of these protective genes, nr4a1, that mutant clone lost its fitness advantage and became less dominant.
“We’ve seen a lot of evidence that the bone marrow is really inflamed in patients with clonal hematopoiesis,” says Zon. “But this is one of the first studies to demonstrate that there are pathways in stem cells that make them resistant to the inflammation.”
Zon and Avagyan believe that taking out that resistance factor could lead to strategies for halting clonal hematopoiesis. If so, this could stop leukemia at its earliest stage.
To that end, Zon and colleagues plan to search for a small-molecule drug that would target nr4a1 or related anti-inflammatory factors. That would be a first step toward a clinical trial aiming to reduce the risk of leukemia in people with clonal hematopoiesis and high-risk mutations. If the team succeeds, the drug would add to the arsenal of drugs the lab has discovered for patients with blood disorders and cancer — thanks to zebrafish.
The current study appears today in the journal Science.
Learn more about research at Dana-Farber/Boston Children’s
Related Posts :
-

Finding new targets for acute myeloid leukemia in children
Acute myeloid leukemia (AML) is the second most common leukemia diagnosed in children. It is hard to treat and can ...
-

Could leukemia be stopped before it starts?
Acute myeloid leukemia (AML), a blood cancer affecting both adults and children, requires more than one genetic “hit” to develop. ...
-

A potential Diamond-Blackfan anemia treatment swims into view
Zebrafish, besides being popular in aquariums, make good stand-ins for studying human diseases. They share about 70 percent of their genes ...
-

In zebrafish, a way to find new cancer therapies, targeting tumor promoters
The lab of Leonard Zon, MD, has long been interested in making blood stem cells in quantity for therapeutic ...