Combining CAR-T cells and inhibitor drugs for high-risk neuroblastoma
Chimeric antigen receptor (CAR)-T cell therapy is a potent emerging weapon against cancer, altering patients’ T cells so they can better find and destroy tumor cells. But CAR-T cell therapy doesn’t work well in every cancer — including many cases of neuroblastoma, a cancer that begins in young children’s nerve tissue and can metastasize to multiple areas of the body.
Neuroblastoma can be deadly: Children with high-risk neuroblastoma have a five-year survival rate of only 50 percent. Roberto Chiarle, MD, at Boston Children’s Hospital and his colleagues now report a way to make CAR-T cell therapy work better in neuroblastoma. They hope to begin testing it soon in children at high risk.
Giving CAR-T cells more targets
Chiarle and colleagues had previously developed CAR-T cells that specifically target the ALK receptor, a known oncogene that drives many cancers. However, not every patient with neuroblastoma has high enough levels of ALK receptors on their tumor cells to attract a strong CAR-T cell attack.
Boosting immunotherapy in lung cancer
The Chiarle lab’s broad focus on anti-ALK immunotherapy also includes the development of a vaccine for non-small-cell lung cancer. Led by postdoctoral fellows Ines Mota, PhD, and Rafael B. Blasco, PhD, the team showed that adding ALK peptides (pieces of the ALK protein) to treatment boosted the immune response against ALK-positive cancer cells and eradicated lung tumors in mice.
Led by research fellow Elisa Bergaggio, PhD, Chiarle’s team tried adding an ALK inhibitor drug to the CAR-T cell treatment. They found that the inhibitor not only silences oncogenic signaling by ALK receptors, but it also increases numbers of these receptors on the cell surface — presenting more targets for CAR-T cells in patients with low ALK expression.
“The ALK receptors are present but inactivated by the ALK inhibitor, and because they’re not active, they stay on the cell surface,” explains Chiarle. “This increased surface expression facilitates CAR-T cell engagement and killing activity.”
Curbing metastatic neuroblastoma
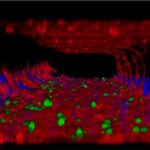
As the team reports in Cancer Cell, the combination of ALK-targeted CAR-T cells and ALK inhibitors proved to be a potent therapy in mice with metastatic neuroblastoma. Mice receiving the combination had significantly reduced tumor growth and better survival.
Chiarle and Bergaggio are now working to create an enhanced, more potent 2.0 version of the therapy. In the meantime, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center has filed for permission from the Food and Drug Administration to test the combination therapy in children with refractory or relapsed neuroblastoma. Susanne Baumeister, MD, of Dana-Farber/Boston Children’s will run the clinical trial, hoped to open this spring.
Children with relapsed or refractory neuroblastoma would first receive escalating doses of ALK-targeting CAR-T cells alone. If this is shown to be safe, the ALK inhibitor would be added. CAR-T cells for each patient will be manufactured in the gene therapy facility at Dana-Farber Cancer Institute.
“Relapsed high-risk neuroblastoma remains a significant clinical problem,” says Suzanne Shusterman, MD, who runs the Neuroblastoma Program at Dana-Farber/Boston Children’s and will be a co-investigator on the trial. “Dr. Chiarle’s preclinical data are very encouraging and we are very excited to have this therapy available to our patients.”
Learn more about the Neuroblastoma Program.
Related Posts :
-

Tackling an aggressive, treatment-resistant lymphoma where it lives
Anaplastic large cell lymphoma, a form of non-Hodgkin lymphoma, is the most common aggressive lymphoma in children. Chemotherapy and radiation ...
-

A potential danger of CRISPR gene editing — and why base editing may be safer
Gene therapy using CRISPR/Cas9 gene editing is currently in clinical trials around the world for a variety of diseases, ...
-

Making ready-made CAR T cells for cancer immunotherapy
In CAR T-cell immunotherapy, T cells from a patient’s own blood are engineered to carry so-called chimeric antigen receptors (...
-

Making immunotherapy safe for AML
Acute myeloid leukemia (AML), the second most common leukemia in children, is hard to treat and has a five-year survival ...