New leads for spinal cord injury: Mapping spinal-projecting neurons in the brain
Only a fraction of people who sustain a spinal cord injury fully regain their motor function. While rehabilitation can help, scientists have long looked for ways to regenerate injured nerve fibers — including, at Boston Children’s Hospital, Zhigang He, PhD, BM.
As part of a collaborative effort by the BRAIN Initiative Cell Census Network, which just published a cell-by-cell “atlas” of the mouse brain, He’s lab took on the ambitious task of profiling those neurons in the brain that send projections to the spinal cord. These neurons, of multiple types, control a variety of bodily functions, including movement. The detailed findings were published last month in Nature.
“Now, we can look at these neurons in terms of their molecular profile and get more insight into what strategy to use to promote regeneration,” He says. “That was one of the major motivations for us to study this.”
A three-pronged system
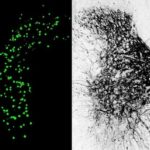
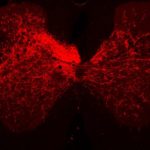
The researchers, led by Carla Winter, an MD-PhD candidate in He’s lab, characterized about 65,000 individual spinal-projecting neurons. They used tools including whole brain imaging, cell labeling techniques, and single-nucleus RNA sequencing to see what genes the cells use and what proteins they make (the transcriptome). They then mapped the identified cell types onto an anatomic and transcriptomic brain atlas created by the Allen Institute for Brain Science.
In all, the team identified 76 types of spinal projecting neurons across the brain. Their functions range from controlling voluntary and involuntary movement to processing sensory inputs to governing autonomic functions like blood pressure, heart rate, and fear responses.
“Molecularly, we’re seeing three divisions of spinal-projecting neurons — three systems for governing different types of movements and functions,” says Winter. These are:
- Division 1 neurons, with just seven cell types arising from three brain regions (the cortex, red nucleus, and cerebellum). All are excitatory, and each projects from a precise brain location to a specific location in the spinal cord, giving these neurons a wide range of functions. “Even though the neurons look similar to one another on a molecular level, we think their functions differ based on their wiring,” says Winter.
- Division 2 neurons, with nearly 50 distinct cell types, are a mix of excitatory and inhibitory. These neurons are scattered throughout the brain, so their projections don’t come in discrete bundles or tracts, and they project to multiple spinal cord locations. The researchers think they may be involved in integrating and coordinating movements.
- Division 3 neurons, a little-known group of neurons with a modulatory function, amplifying or quieting down entire brain-spinal circuits. The researchers documented 20 different neuron types in the hypothalamus, midbrain, and brainstem. “These are different from typical neurons, and act via slow neurotransmission or neuropeptides,” says Winter.
Clues to spinal cord regeneration?
The team had to overcome a number of technical challenges, including isolating neurons whose projections, known as axons, can be several feet long in humans. The detailed molecular profiles offer many opportunities to explore new treatment possibilities for neuromotor conditions, including spinal cord injury.
Related: A study in Nature Neuroscience, led by Aritra Bhattacherjee, PhD, and Yi Zhang, PhD, in the Program in Cellular and Molecular Medicine at Boston Children’s, recently identified distinct cell types and circuits in the brain’s prefrontal cortex that underlie neuropathic pain.
“We usually consider growth factors to promote axon regeneration, but in the past we didn’t know what growth factor receptors specific populations of neurons express,” says He. “Now, this becomes very clear.”
The study also adds valuable information to the Allen Brain Atlas, filling in some neuron types not previously recognized and where their axons go. In the future, the researchers hope to study spinal projecting neurons in the brains of nonhuman primates and humans. They believe these neurons have some features in common with the mouse brain, but also more organizational complexity.
Related Posts :
-

Scar-free healing after spinal cord injury relies on specialized cells
Scar tissue prevents nerves from communicating with each other. Microglia cells in the central nervous system help prevent scar tissue ...
-

Discoveries promise new strides for spinal cord injury patients
When neurobiologist Clifford Woolf, MB, BCh, PhD, began investigating potential treatments for spinal cord injury more than 30 years ago at ...
-

Inhibiting inhibitory neurons gets mice with spinal cord injury to walk again
Most people with spinal cord injury are paralyzed from the injury site down, even when the cord isn’t completely ...
-

Novel therapeutic cocktail could restore fine motor skills after spinal cord injury and stroke
Neuron cells have long finger-like structures, called axons, that extend outward to conduct impulses and transmit information to other neurons ...