One-time treatment could block a deadly form of graft-versus-host disease
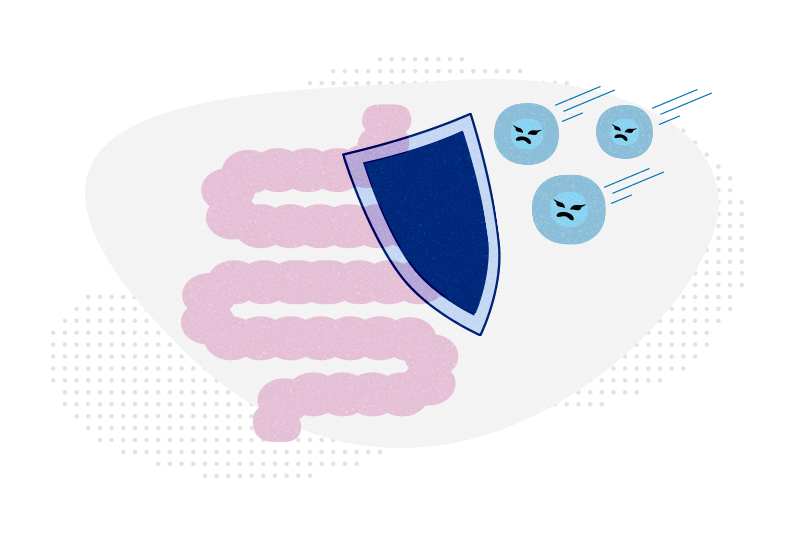
Even when a bone marrow transplant cures leukemia or lymphoma, patients can still pass away from graft-versus-host disease (GVHD), in which T cells in the donor graft attack the recipient’s own tissues. Leslie Kean, MD, PhD, director of stem cell transplant at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, has long sought to prevent this fatal complication.
Now, Kean and her collaborators Victor Tkachev, PhD, at Mass General Brigham; Ivan Maillard, MD, PhD, at the University of Pennsylvania; and scientists at Regeneron Pharmaceuticals describe a treatment that could prevent intestinal GVHD — the most severe form of this disease — while preserving the immune system’s ability to fight infections and cancer. Their findings were published in Science Translational Medicine and a clinical trial is now in the planning stages.
Stopping intestinal GVHD before it starts
Nearly all cases of severe, acute GVHD affect the gastrointestinal tract, and such cases are especially deadly. The new approach prevents the donor’s T cells from ever migrating to the intestine by inhibiting Notch signaling, a cell signaling system found in all animals. It could potentially be delivered as a one-time treatment before a bone marrow transplant.
“This would be the first strategy for GVHD that affects the priming of T cells right at the time they’re being activated, with the probability of being less globally immunosuppressive because it then selectively blocks their migration to the gut,” says Kean.
Maillard had previously shown over more than a decade of work in mouse models that Notch signaling promotes the donor T-cell attack against transplant recipients. Kean, who specializes in transplant immunology in large animal models, approached Maillard after attending one of his talks. Kean told Maillard that she was beginning to notice a strong Notch signal in her data as well. That initial conversation sparked a seven-year collaboration that validated and expanded upon Maillard’s findings in non-human primates, whose immune system closely resembles that of humans.
The researchers showed that the Notch pathway is evolutionarily very similar across species. For this reason, Kean and Maillard predict that Notch-directed medicines could be effective in preventing GVHD in patients.
Repurposing an antibody
Kean, Tkachev, and Maillard confirmed that Notch signaling exerts most of its effects via the Delta-like Notch ligand, or DLL4. Notch in the donor T cells interacts with DLL4 in the transplant recipient. Blocking DLL4 — with an antibody developed at Regeneron — inhibits a specific pathway involved in intestinal infiltration, the researchers found. As a result, harmful donor T cells never migrate to the intestine.
The antibody blocking DLL4 was originally developed to inhibit blood vessels in tumors. Regeneron has collaborated with the Kean and Maillard teams to explore repurposing it as a means of preventing GVHD. As evidence of the power of this approach, Kean and Tkachev note that most of the donor-recipient pairs in their animal models were very poorly matched immunologically.
“Our experiments used DLL4 blockade alone, without requiring any other immunosuppression,” adds Maillard. “Our results were all based on making the graft less capable of inducing GVHD.”
Clinical trial in high-risk leukemia
The planned clinical trial of anti-DLL4 would be a follow-on study to an ongoing trial led by John Koreth, MD, PhD, of Dana-Farber. Subjects would be adults with relapsed or refractory acute myeloid leukemia (AML) and/or myelodysplastic syndromes who are receiving haploidentical transplants with no immunosuppressive drugs. Because the probability of relapse is high in these patients, many currently aren’t considered eligible for bone marrow transplants.
“It has been rewarding to see all our work on Notch signaling come to fruition in this collaboration,” says Maillard. “We have learned a lot about how Notch-targeting drugs might best be deployed to help patients.”
Kean is excited by the prospect of making bone marrow transplants safer and more accessible.
“My career has been focused on improving outcomes of transplant,” she says. “The field has a growing number of possibilities to achieve this goal as new targeted treatments come online.”
The work was funded by the Leukemia & Lymphoma Society, the National Institutes of Health, Regeneron, Be The Match Foundation/CIBMTR, and the American Society for Transplantation and Cellular Therapy. Tkachev was co-first author on the paper along with PhD candidate Ashley Vanderbeck and Eric Perkey, MD, PhD from Maillard’s group at the University of Pennsylvania. See the paper for a complete list of authors and competing interests.
Learn more about the Stem Cell Transplant program at Boston Children’s.
Related Posts :
-

Surviving stem cell transplant: New hope when the donor isn’t a full match
To see Tara Daniels today, with a corporate job in marketing and about to close on a house, you’d ...
-

An unexpected journey reveals a potent way to attack tumors
Research on the effects of prenatal exposure to the Zika virus has yielded an unexpected dividend: a potentially promising way ...
-

How a leukemia hijacks the genes needed by blood stem cells
As a child, Lynn Aureli didn’t know that a particular genetic change contributed to her acute myeloid leukemia (AML) — ...
-

Building better antibodies, curbing autoimmunity: New insights on B cells
When we’re vaccinated or exposed to an infection, our B cells spring into action, churning out antibodies that are ...