Precision chemo-immunotherapy for pancreatic cancer?
Pancreatic cancer is highly lethal and in great need of better treatments. Only about 10 percent of patients remain alive five years after diagnosis. In a new study, researchers in the lab of Marsha Moses, PhD, at Boston Children’s Hospital offer a glimmer of hope.
Key takeaway
An antibody-drug combination effectively targeted, penetrated, and shrank pancreatic tumors in mice.
Using a highly selective, potent, engineered antibody-drug combination, they produced marked and lasting tumor regression in a mouse model of pancreatic cancer.
The preclinical study provides the basis for further work to advance the approach to the clinic, Moses says.
Findings appear this week in Advanced Science.
Penetrating pancreatic tumors
Pancreatic cancer has proven very difficult to treat with drugs. The tumors have a limited blood supply, making delivery of drugs a challenge. Moreover, a dense connective tissue called stroma surrounds and protects them.
“It can be difficult to get drugs into these tumors,” says Moses, who directs Boston Children’s Vascular Biology Program. “We developed a novel chemo-immunotherapy agent that selectively recognizes and penetrates pancreatic tumors better than other therapeutics.”
The precision of our approach comes from both the specific targeting and the ability to monitor that targeting with MRI.”
– Marsha Moses
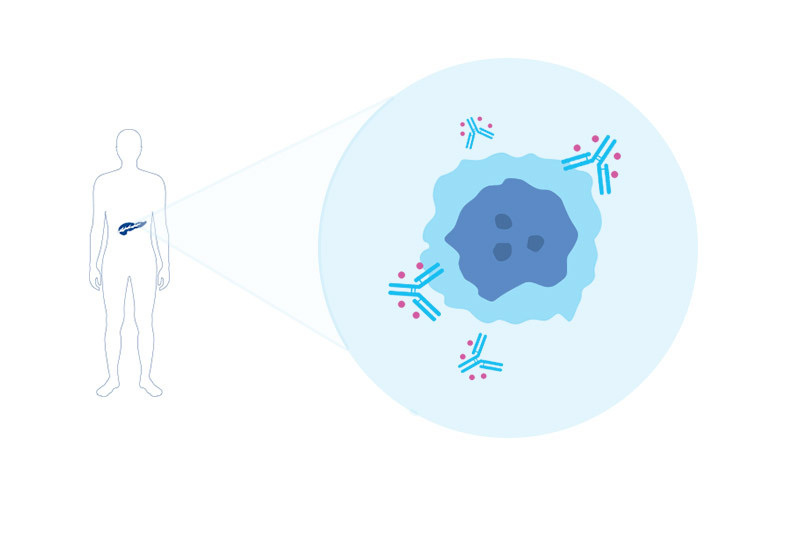
Led by Jing Huang, PhD, the paper’s first author, the researchers developed a “smart” antibody-drug conjugate, or ADC. It consists of two parts: an antibody that selectively homes to a molecule on the surface of pancreatic cancer cells, known as ICAM1, and a drug toxic to cancer cells. The drug kills cells bearing ICAM1 on their surface, while sparing normal cells.
“The size of the ADC is similar to the size of a single antibody: less than 10 nanometers,” says Peng Guo, PhD, of the Moses Lab, co-senior author on the paper. “Because of this ultra-small diameter, it can penetrate the stroma and reach pancreatic tumor cells better than other novel treatments such as T-cell immunotherapy or nanomedicines.”
Rational selection of antibody and drug
The team chose ICAM1 as a target for their ADC after screening the tumor surface for dozens of different proteins. In 2014, the Moses lab showed high levels of ICAM1 on triple-negative breast cancers. ICAM1 is also abundant on melanoma and thyroid cancers. To find the best drugs for their ADC, the team performed a similar unbiased screen, drawing from anti-cancer drugs already used clinically.
Next, the researchers tested four different ADCs in two human pancreatic cancer cell lines and in normal pancreatic cells. The ADC that came out on top combined ICAM1 antibodies with the cytotoxic drug DM1 (mertansine), used in HER2-positive breast cancer. It killed the tumor cells most effectively, but did not harm non-cancerous pancreatic cells, which do not produce ICAM-1.
Shrinking pancreatic tumors
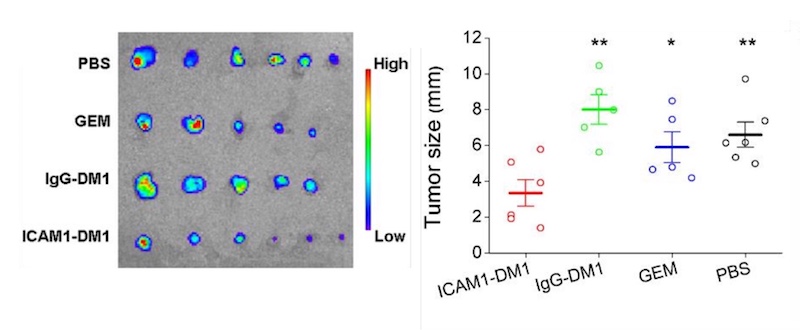
Finally, the team randomized mice with pancreatic tumors to receive one of four treatments: the ICAM1-DM1 antibody conjugate; DM1 bound to a non-targeting antibody (IgG); gemcitabine, a first-line chemotherapy drug used in pancreatic cancer; or a sham treatment. All were given as systemic injections.
Compared with the other groups, mice receiving the DM1-ICAM1 antibody conjugate had a significant reduction in tumor size. The reduction persisted during the 14-week study, even after just two doses. The treatment also effectively inhibited metastasis to normal organs including lung, liver, and spleen. The researchers did not find any toxicity, assessed by weighing the animals and examining their organs.
Noninvasive tumor monitoring
To complement the ICAM1 ADC therapy, Huang developed a molecular tumor imaging technique that uses MRI to confirm the presence of ICAM1 on the tumor. Such imaging could potentially help to predict the treatment’s effect and monitor changes over time, without the need for an invasive biopsy. Eventually, Moses hopes to be able to monitor treatment effects using two urine biomarkers her team previously reported.
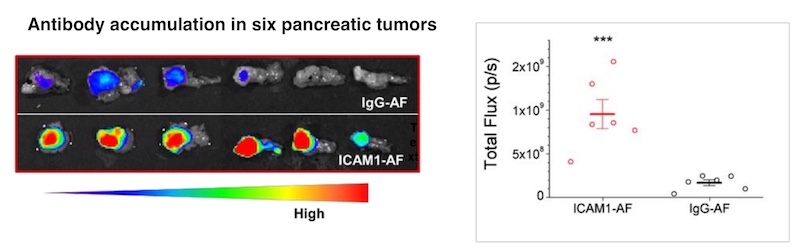
While other ADCs have been tested in pancreatic cancer, none have proved effective enough in patients, and they have also caused off-target toxicity, says Moses. “The precision of our approach comes from both the specific targeting and the ability to monitor that targeting with MRI,” she says.
The ICAM1-DM1 ADC adds to a larger portfolio of targeted, patented cancer drug delivery systems being developed in the Moses lab. Others include nanolipogels, liposomes, and exosomes.
Agoston T. Agoston, MD, PhD, of Brigham and Women’s Hospital was a coauthor on the paper. The Advanced Medical Research Foundation and the Karp Family Foundation supported the study.
Explore the Vascular Biology Program at Boston Children’s Hospital.
Related Posts :
-

A true hero’s journey: How a team approach helped Wolfie overcome pancreatitis
Wolfgang, affectionately known as “Wolfie,” is a bright and energetic 7-year-old with a quick wit and a love for making ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

A toast to BRD4: How acidity changes the immune response
It started with wine. Or more precisely, a conversation about it. "My colleagues and I were talking about how some ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...