Platelet profiles could enhance care for bleeding disorders, cardiovascular disease, cancer
The trillion-odd platelets in our blood are best known for controlling bleeding, helping clots form when we sustain an injury. But platelets can also go rogue. They contribute to clotting in cardiovascular disease and diabetes, leading to strokes and heart attacks, and can be hijacked by cancers to help them spread.
Key takeaways
A new platelet profiling technology could help guide care in a broad range of disease settings.
In the first use case, a clinical study is testing platelet profiles as a way to predict bleeding risk in immune thrombocytopenia (ITP).
As Ben Spurgeon, PhD, has been finding, not all platelets are alike — they carry different “portfolios” of molecular markers that reflect their different roles and could potentially predict disease.
“Until recently, our ability to identify different platelet subsets was limited by technology,” he says. “Now, we can measure many biomarkers on the surface of a platelet. By looking at combinations of these biomarkers, we can identify platelet subsets that we think drive specific diseases.”
Spurgeon, a fellow in Boston Children’s Center for Platelet Research Studies, led by Andrew (Larry) Frelinger, PhD, has been delving into platelets for several years. Frelinger’s team has patented a next-generation technology that, according to Spurgeon, provides significantly more information than existing platelet tests, all from a single drop of blood.
That technology has drawn interest from clinicians across the country who are seeking better tests for diseases, from breast cancer to heart attack to bleeding disorders.
Platelet Xploration
Standard platelet tests use flow cytometry, in which blood bound to fluorescently labeled antibodies is flowed past a laser. Platelets are then classified based on how they fluoresce and scatter light. The new technology, mass cytometry, uses antibodies that have been tagged with different metals and that bind to specific biomarkers on the platelet surface. Blood is flowed through a mass cytometer, which evaluates platelets one at a time (about 1,000 per second) with mass spectroscopy to quantify the amount of each metal bound to each platelet.
“If more of a metal is detected, it means levels of that biomarker are higher,” Spurgeon explains.
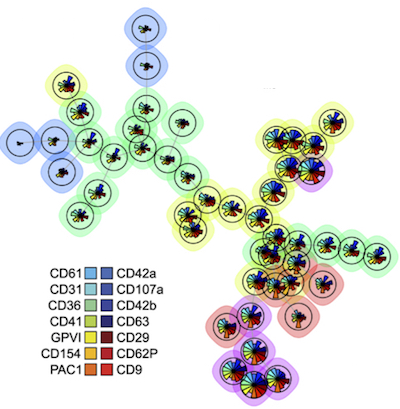
With the help of a machine learning algorithm, Spurgeon and his colleagues have identified more than 20 distinct surface marker profiles, each identifying a novel subset of platelets. They hypothesize that each profile reflects differences in platelet activity and how they interact with factors in the blood and blood vessels.
“We want to see if specific platelet subsets are altered in different diseases,” says Spurgeon. “If they are, it raises the possibility that they could serve as biomarkers of disease or predictors of clinical outcomes.”
Belén Vicente, a biotechnologist and MBA candidate at MIT Sloan School of Management, joined the team to help validate and market the technology and get it into the clinic as a diagnostic test. Through the Harvard/MIT Activate program, she and Spurgeon proposed the formation of a startup, Platelet Xploration, which was a semifinalist in two recent competitions — the MIT $100K and the CYTO Innovation showcase.
First use case: Platelet profiles in immune thrombocytopenia
For their first clinical project, the team is developing a better platelet test for bleeding risk in immune thrombocytopenia (ITP). In this rare autoimmune bleeding disorder, the bone marrow produces fewer platelets than normal, and the body destroys them even as they’re being made.
About 10 to 20 percent of children with ITP present with bleeding symptoms (urinary, or from the nose or mouth) that indicate the need for platelet-raising therapy. In rare cases, ITP can cause bleeding in the brain, and even more rarely, bleeds can be fatal. But in most children, ITP causes only skin bruising and often goes away on its own.
“Many children with ITP and low platelet counts do not have significant bleeding,” says Rachael Grace, MD, MMSc, a hematologist with the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.

The problem is knowing who and when to treat. Current platelet tests, which look mainly at numbers of platelets, don’t reliably predict a child’s risk. Out of caution, clinicians tend to treat ITP more aggressively than they need to, using steroids, IV immunoglobulin, and sometimes other agents to stimulate platelet production or suppress the immune system. The treatments raise platelet counts, but they don’t cure ITP and their side effects can be worse than the disease.
About 10 years ago, Grace formed the ITP Consortium of North America (ICON) to foster large-scale collaborations and gain a better understanding of how to approach this rare illness.
“We’re often faced with the question: should we treat with medications, or closely observe? We want to follow the path of least harm,” says Grace. “If you’re not used to managing ITP, this decision can be challenging.”
Not all platelets are the same
Grace, Frelinger, and their colleagues decided to look for platelet parameters other than platelet count that might correlate with bleeding symptoms. Their first study, involving 57 patients at Boston Children’s, found that certain markers of platelet activation were associated with more severe bleeds, as was having more immature platelets.
Grace and other members of ICON are now collaborating with Spurgeon and Frelinger on a new clinical study, funded in part by a grant from the Platelet Disorder Support Association. The study will add 15 new platelet markers measured by mass cytometry and machine-learning profiling of platelet subsets. This study will enroll patients at six medical centers; the researchers expect results in 2021.
“If we knew who is likely to have future bleeding, we’d know who to treat with medication,” she says. “What we learn from this study could also apply to older patients with ITP.”
Platelet prognostications
Meanwhile, Spurgeon, Vicente, and the Center for Platelet Research Studies are pursuing other uses for platelet profiling. One project is seeking to develop a “liquid biopsy” for breast cancer based on platelet profiles, potentially avoiding some invasive breast biopsies.
“Breast cancers and other solid tumors can hijack and reprogram platelets, both in the blood and as they’re being produced in the bone marrow, giving rise to rogue platelet subsets that potentially reflect the cancer’s stage and severity,” explains Spurgeon. “These platelets release factors that help the tumor spread and grow, and might help mask cancers from the immune system.”
Breast cancers and other solid tumors can hijack and reprogram platelets, both in the blood and as they’re being produced in the bone marrow, giving rise to rogue platelet subsets.”
– Ben Spurgeon
Additionally, Spurgeon, and Frelinger are collaborating with Boston Children’s neonatologist Martha Sola-Visner, MD, who has found that newborns’ platelets function differently and wants better measures to guide transfusion decisions. Their project has found platelet subsets that are unique to cord blood that might have relevance for newborn care.
Another potential project may explore the association between platelet characteristics and outcomes of heart attack and stroke.
All told, the team estimates that up to 50 million tests could be performed each year in the U.S. using their technology, in a broad range of disease settings.
“It has generated excitement from many clinicians we’ve spoken to,” says Spurgeon. “They’re eager to see how this test performs in their patients and how the results can be used to improve patient care.“
More research and innovation from Dana-Farber/Boston Children’s
Related Posts :
-

Tough cookie: Steroid therapy helps Alessandra thrive with Diamond-Blackfan anemia
Two-year-old Alessandra is many things. She’s sweet, happy, curious, and, according to her parents, Ralph and Irma, a budding ...
-

Unique data revealed just when Mickey’s heart doctors could operate
When Mikolaj “Mickey” Karski’s family traveled from Poland to Boston to get him heart care, they weren’t thinking ...
-

It’s all in the PV loops: New analytical model could improve circulation assessments before heart surgery
The double-switch operation corrects the congenital reversal of the heart’s ventricles and its two main arteries. It’...
-

Blood across our lifetimes: An age-specific ‘atlas’ tells a dynamic story
The stem cells that form our blood, also known as hematopoietic stem cells (HSCs), are with us throughout our lives. ...





