Guidance for assessing treatment response in pediatric brain tumors
Assessing patients’ response to cancer therapy can be challenging, especially in neuro-oncology. Generally, we assess treatment response by a change in tumor size on MRI scan. However, with brain tumors, changes on MRI scan can be difficult to interpret. A decrease in tumor size may indicate treatment is having an effect; however, a drug can be active without changing the size of the tumor, or a tumor can appear larger in response to some therapies, particularly immunotherapy, a phenomenon known as “pseudoprogression.”
· Three reports detail guidelines for assessing treatment response in children with low-grade glioma, high-grade glioma, and DIPG.
· The guidelines will be incorporated into clinical trials to be prospectively evaluated and validated.
The Response Assessment in Neuro-Oncology (RANO) Working Group formed to address these issues and recommend appropriate endpoints for clinical trials. The group includes international experts in neuro-oncology, neuro-radiology, radiation oncology, neurology, and neurosurgery. Recognizing the unique issues in children with central nervous system tumors, a companion group formed, called Response Assessment in Pediatric Neuro-Oncology (RAPNO), which I chair.
Our goal was to establish criteria for treatment response in children with CNS tumors that can be prospectively evaluated in clinical trials. In 2018, RAPNO published recommendations for medulloblastoma response assessment in Neuro-Oncology. Its most recent guidelines, published in three articles in The Lancet Oncology this month, focus on gliomas.
Low-and high-grade gliomas and DIPG: Assessing tumor and patient response
For pediatric low-grade gliomas, the most common CNS tumors in children, recommendations include imaging response assessments, and offer additional guidelines for measuring visual functional outcomes in patients with optic pathway tumors. RAPNO’s low-grade glioma committee was led by Jason Fangusaro, MD, of Children’s Healthcare of Atlanta and Olaf Witt, MD, of Deutsches Krebsforschungszentrum.

For pediatric high-grade gliomas, a leading cause of cancer-related death in children, RAPNO’s recommendations for response assessment include the use of MRI of the brain and the spine, clinical evaluation, and potentially the use of corticosteroids or antiangiogenics. RAPNO also defined imaging standards for the brain and spine. In contrast to adult high-grade glioma, the pediatric recommendations include diffusion-weighted imaging and a higher reliance on T2-weighted fluid-attenuated inversion recovery. The high-grade glioma committee was led by Craig Erker, MD, of Dalhousie University and Michael Prados, MD, of UCSF.
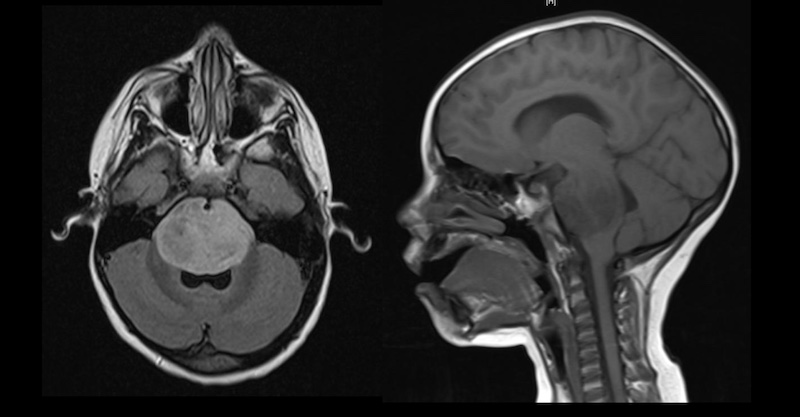
For diffuse intrinsic pontine glioma (DIPG), an aggressive brainstem tumor, response recommendations include assessment using MRI of brain and spine, neurological examination, and anti-inflammatory or antiangiogenic drugs. The guidelines define clinical imaging standards for these difficult-to-measure tumors. Tabitha Cooney, MD, of Dana Farber Cancer Institute and Ken Cohen, MD, of Johns Hopkins led our DIPG committee.
Related:
Unlocking a treatment for diffuse intrinsic pontine glioma
A family pours its grief into supporting research on gliomatosis cerebri
These guidelines are based on published, peer-reviewed science (when available), expertise of the committee members, and consensus within the groups. They will now be incorporated into clinical trials to be prospectively evaluated and validated. These recommendations will harmonize evaluations of children with brain tumors in clinical trials, leading to more valid comparisons between trial outcomes, and offering indicators of meaningful clinical benefit.
About our blogger: Katherine Warren, MD, is clinical director of pediatric neuro-oncology at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
Visit the Brain Tumor Center at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
Related Posts :
-

A toast to BRD4: How acidity changes the immune response
It started with wine. Or more precisely, a conversation about it. "My colleagues and I were talking about how some ...
-

“A setback for a comeback”: Brody perseveres with Paget-Schroetter Syndrome
Baseball has been part of Brody Walsh’s story from the very start. Now 19 and a college sophomore, Brody pitches ...
-

Tough cookie: Steroid therapy helps Alessandra thrive with Diamond-Blackfan anemia
Two-year-old Alessandra is many things. She’s sweet, happy, curious, and, according to her parents, Ralph and Irma, a budding ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...