Mouse/human model provides new way to study neuroblastoma
Neuroblastoma is a rare childhood cancer affecting about 800 children each year in the United States. Because of its unusual behavior — tumors in infants often disappear spontaneously without treatment while it can be aggressive and fatal in toddlers — studying the disease has been complicated.
That may change with a new research tool: a mouse genetically altered to develop and grow human neuroblastoma tumors. Known as a chimera, with a combination of mouse and human genes, the model offers a unique way to study human neuroblastoma tumors, particularly the interactions of the immune system with the tumors. The research was published in a paper in Cell Stem Cell.

“Neuroblastoma is known as an immunologically cold tumor, which means that therapies that target the immune system, like anti-PD1, anti-PDL1, and anti-CTLA4 therapies, are not very effective,” says co-senior author on the paper, Rani George, MD, PhD, a neuroblastoma researcher and physician at the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center. “With this model, we will now be able to study how the disease evolves from the very beginning and how it interacts with the immune system to develop better treatments, including better responses to immune treatments.”
At the heart of the technique is the injection of human neuroblastoma-promoting genes for neuroblastoma into a healthy mouse with an intact immune system, yet is tolerant to human neuroblastoma cells.
“It is really the first model for tumor-formation in an immunocompetent mouse,” says George. “This allows us to study the interaction between the tumor and its microenvironment and to study human neuroblastoma development in an immune competent setting.”
Human neuroblastoma genes inserted
The research team first engineered human stem cells to express two genes known to be abnormal in neuroblastoma, MYCN and mutated ALK. Next, they modified the cells to develop into neural crest cells, the cells from which human neuroblastomas are derived. Then, they injected these cells into mouse embryos. As the mouse embryos developed, human tissue containing these neuroblastoma-promoting genes proliferated throughout the animal’s neural crest. Finally, the team triggered the cancer genes with doxycycline, causing development of neuroblastoma tumors in some mice.
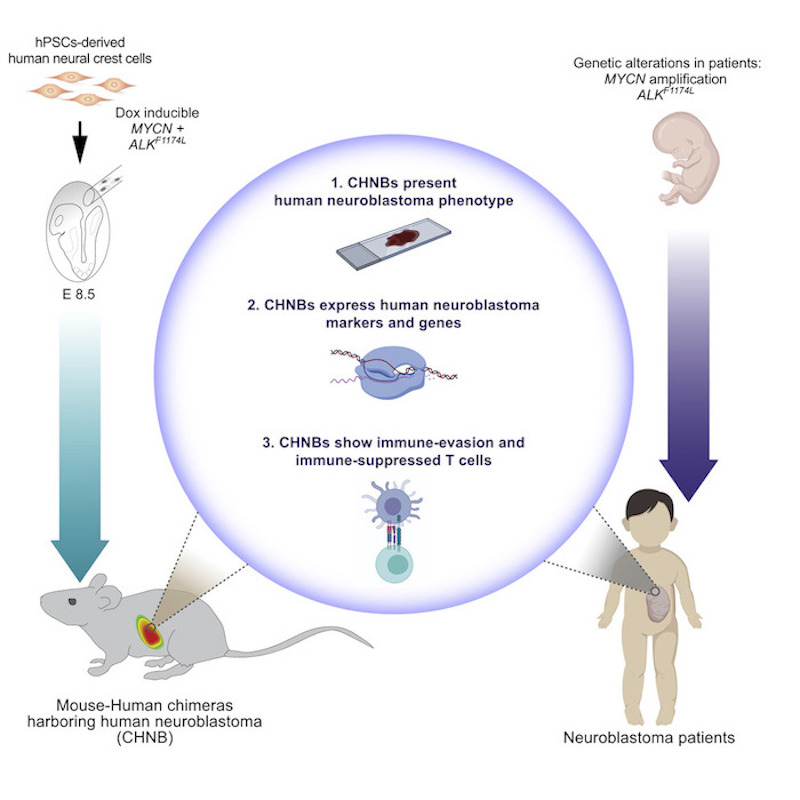
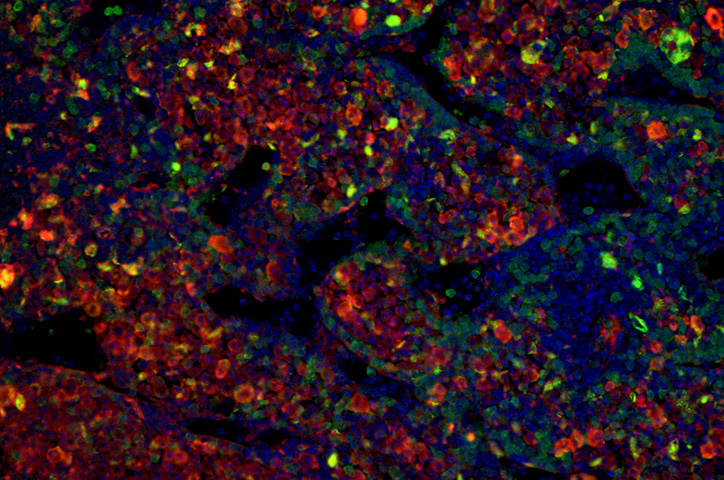
Over 15 months, 14 percent of the mice developed tumors — 29 mice out of 198. The tumors mostly appeared in the space behind the abdominal cavity close to the nerves along the spinal cord, although one mouse developed a tumor in its adrenal gland. Both locations are common places where children develop neuroblastoma.
Samples from the tumors confirmed their human origin. Growth patterns of the mouse neuroblastoma cells resembled human neuroblastoma cells. They also contained cell markers typical of human tumors, and some grew in characteristic rosette shapes. RNA-sequencing revealed that chimeric tumors presented similar gene expression profiles to human neuroblastomas.
Equivalent immune system response
Until now, researchers lacked a reliable mouse model for studying neuroblastoma tumors. Mice could be genetically engineered with cancer-causing genes, but they developed mouse tumors, not human cancers. Established human tumors could be implanted in mice, but this required the use of an immunocompromised mouse, utterly defeating the goal of studying how these cancers develop and interact within a healthy immune system.
In this study, the chimeric mice showed a clear anti-tumor immune response against the human neuroblastoma tumors, resembling that in neuroblastoma patients. The model also mimicked how human neuroblastoma cells can out-maneuver the immune response by evading T-cells. They produce chemicals that “exhaust” T-cells, thereby weakening their ability to attack invading cancer cells.
Now that the model has been created and validated, researchers plan on using it to find new neuroblastoma treatments. George plans on using the mouse/human neuroblastoma chimera to study how genes that trigger cancers like neuroblastoma evolve during development. But she has a longer-term vision, too.
“Eventually, our ultimate goal is to be able to take a patient’s tumor cells, make the tumor grow in the mouse, and study the unique immune interactions so that we can gear treatment to each patient.”
This research was produced from a collaboration between Malkiel A. Cohen, Shupei Zhang, Haiting Ma, George Bell, and Rudolf Jaenisch at the Whitehead Institute for Biomedical Research; Satyaki Sengupta, Bandana Sharma, and Rani George at Dana-Farber/Boston Children’s Blood and Cancer Center; and Brendan Horton and Stefani Spranger from the Koch Institute for Integrative Cancer Research.
Research funding was provided by the National Institutes of Health, as well as grants from the Emerald Foundation, the LEO Foundation, the Melanoma Research Foundation, and the St. Baldrick’s Foundation.
Related Posts :
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...
-

“A setback for a comeback”: Brody perseveres with Paget-Schroetter Syndrome
Baseball has been part of Brody Walsh’s story from the very start. Now 19 and a college sophomore, Brody pitches ...





