Gene therapy for hearing loss: Tag-teaming from the lab to the clinic
One-year-old Miles Mahota is one of the first patients with hereditary hearing loss to receive gene therapy at Boston Children’s Hospital — to striking effect. In May, Miles received an injection delivering a healthy copy of a gene called otoferlin into the cochlea of his inner ear.
Now, Miles is thriving. He’s had no adverse events, and his hearing has already improved remarkably — at long last, he’s responding to his parents’ voices. Testing shows dramatic gains, and with the full effect of the therapy expected in three to four months, there’s still more progress ahead.
Made possible, in part, by innovations at Boston Children’s, Miles’ treatment is just the first in a pipeline of gene therapies for hearing loss, thanks to a unique partnership between Boston Children’s otolaryngologists and laboratory scientists. The team hopes to advance several other gene therapies to clinical trials over the next decade.
“How we discuss hearing loss with our patients and families is different even from two years ago,” says Eliot Shearer, MD, PhD, an otolaryngologist and site leader for two separate otoferlin trials at Boston Children’s. “There are now more options and it informs our decisions.”
Shearer sees Boston Children’s — with its large cohort of thousands of patients with genetic hearing loss, a robust group of science discovery labs, and a clinical trials group eager to offer patients new options — as an ideal trial site for gene therapies.
The benefits of gene therapy
Up to 60 percent of children born with hearing loss have an identifiable genetic cause. “We started doing genetic testing for hearing loss in the late 1990s,” says Margaret Kenna, MD, MPH, who directs clinical research in Boston Children’s Department of Otolaryngology and Communication Enhancement. “Connexin 26 (GJB2) was the first gene, and we were one of the first places in the country to test for it.”
To date, mutations in at least 150 genes have been implicated in hearing loss. Each disrupts hearing differently. If gene therapy is done early, in the first one to four years of life, children can acquire normal spoken language and connect better with others socially. They can also avoid the need for cochlear implants, which have been life-changing but can stop working, get infected, or otherwise need replacement. “The ideal would be to do gene therapy once and cure hearing forever,” says Kenna.
Gene therapy can also potentially restore a more natural kind of hearing. “The inner ear has thousands of cells tuned to different frequencies that allow us to hear,” notes Shearer. “A cochlear implant only has about 20 different electrodes.”
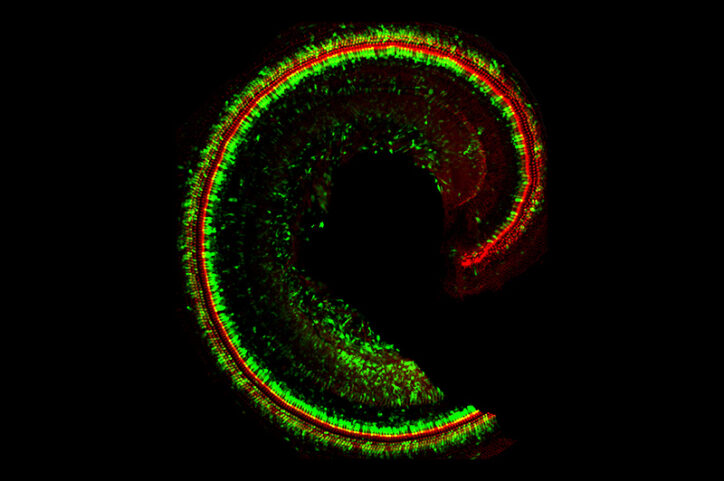
TMC1 and “Beethoven” mice
Scientist Jeffrey Holt, PhD, has studied the inner ear and explored gene therapies for hearing loss for the past 25 years. Holt and collaborators put a stake in the ground in 2011, showing that two related proteins, TMC1 and TMC2, are essential for hearing. The proteins sit atop sensory hair cells in the cochlea. When sound waves vibrate the hairs, TMC1 and TMC2 let calcium into the cells, creating electrical signals that our brains interpret as sound. Holt showed that when the TMC1 gene is mutated, this calcium influx is reduced or lost, weakening hearing.
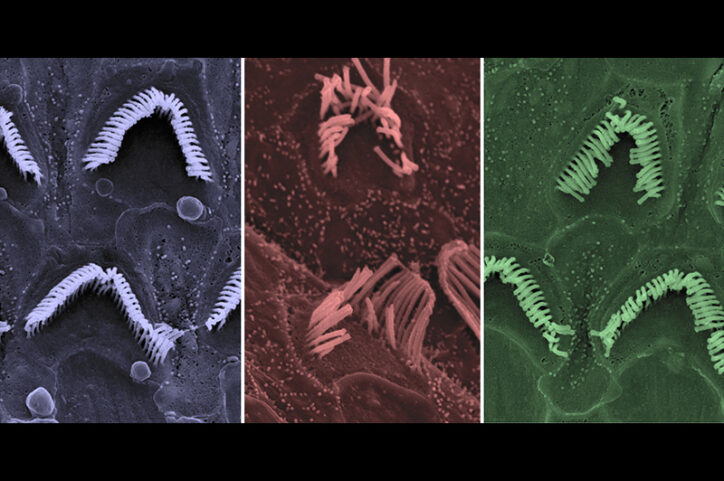
More than 70 different TMC1 mutations have been identified in humans. In 2015, Holt and colleagues showed that gene therapy providing a healthy copy of TMC1, could restore hearing in deaf mice. They used a harmless engineered adeno-associated virus, called a vector, to deliver the gene, adding a genetic sequence that turns the genes on in sensory hair cells.
“Our work was the first to show you could use a viral vector to put DNA into a sensory hair cell in the inner ear,” Holt says.
Holt’s team later showed that CRISPR-Cas9 gene editing can prevent hearing loss in “Beethoven “mice, which carry dominant TMC1 mutations, and that base editing, a more precise technique, could also repair TMC1 and restore partial hearing.
Stereocilin and a strategy for delivering large genes
Holt’s lab next explored gene therapy targeting stereocilin, a gene implicated in 15 to 20 percent of genetic hearing loss. Stereocilin enables sensory hair cells to stand tall in a bundle so they can physically touch the ear’s tectorial membrane — and thereby pick up sound vibrations.
“If stereocilin is mutated, you don’t have that contact, so the hair cells are not stimulated properly,” Holt explains.
There was one challenge: Stereocilin is a very large gene, too large to fit into a viral vector. Holt’s solution was to divide it between two vectors. In tests, the dual vectors improved hearing in mice with severe hearing loss — sometimes to normal levels of hearing.
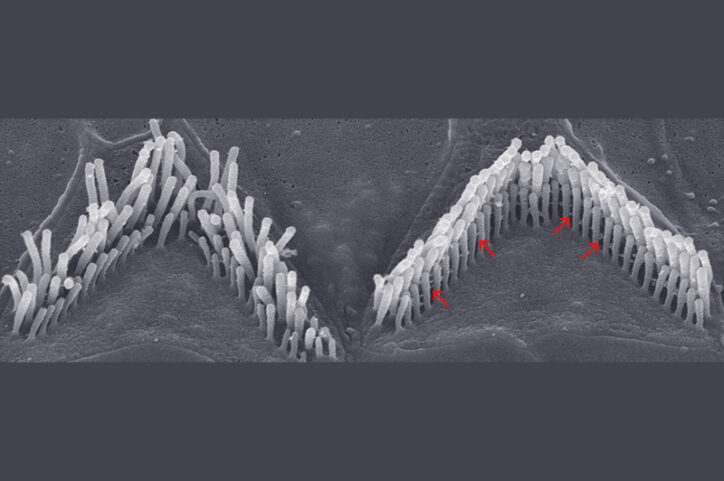
A similar two-vector strategy is now being used in both otoferlin trials — and in preclinical studies of several other therapies delivering very large genes.
A lab-to-clinic pipeline
Holt and Boston Children’s are currently in discussions with potential industry partners to move TMC1 and stereocilin gene therapy into clinical trials. In the meantime, the lab of Holt and Gwenaelle Géléoc, PhD, is developing various other gene therapies in partnership with Karl Koehler, PhD and the clinical otolaryngology team.
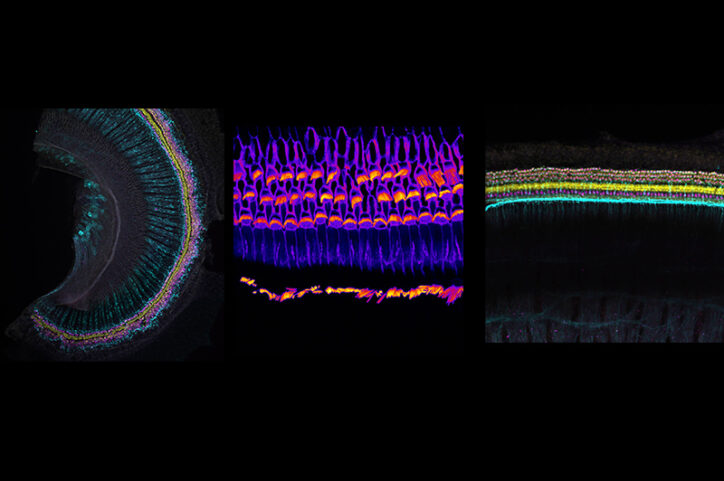
To advance these efforts, Shearer, Kenna, and genetic counselor Shelby Redfield, MS, CGC have established a Translational Hearing Genomics Lab and a biobank of stem cells from children with genetic hearing loss. One gene of interest is TMPRSS3, which causes hearing loss in thousands of patients. Working with a young adult with a TMPRSS3 mutation and partial hearing loss, Shearer and Koehler are testing potential treatments in mice and inner-ear organoids made from her stem cells. Shearer hopes to develop a fast-track clinical protocol to test the most promising approach in his patient in collaboration with Timothy Yu, MD, PhD, in the Division of Genetics and Genomics, who has pioneered custom genetic treatments using antisense oligonucleotides (ASOs), a pharmaceutical genetic therapy.
Another team effort is focused on Usher syndrome, one of the most common forms of deaf-blindness caused by at least nine different genes. Using mice and inner-ear and retinal organoids — developed by Koehler’s lab — Géléoc and Holt are testing gene therapies and ASOs that could repair each Usher patient’s genetic mutation.
Prenatal gene therapy to prevent hearing loss?
The original connexin 26 (GJB2), implicated in up to 30 percent of cases of inherited deafness, turns out to be harder to correct: It causes the sensory hair cells to die off before they can be repaired with gene therapy.
But Shearer envisions that someday this gene — and others that cause early damage — could be repaired before a child is born. Many hearing-related genes are included in prenatal testing, and the otolaryngology team already receives referrals from Boston Children’s Fetal Care and Surgery Center.
“For me,” says Holt, “it is amazing to see my life’s work finally make its way into the clinic.”
Refer a patient to the Department of Otolaryngology and Communication Enhancement, or email Eliot Shearer, MD, PhD to learn more about the OTOF gene therapy trial.
Related Posts :
-

Optimized CRISPR/Cas9 gene editing averts hearing loss in 'Beethoven' mice
Using a novel gene-editing approach, scientists at Boston Children’s Hospital and Harvard Medical School have salvaged hearing in a ...
-

A different kind of hearing: Caleb’s cochlear implant
Caleb recently told his mother, “I think I’m going to write my own stories.” This news didn’t surprise ...
-

‘Empowered to be there for Teagan’: New parents learn about hearing loss
Teagan O’Brien is a bright, spunky 4-year-old who loves reading, dancing, and playing outdoors. Her parents, Kim and Donnie, ...
-

A sickle cell first: Base editing, a new form of gene therapy, leaves Branden feeling ‘more than fine’
Though he doesn’t remember it, Branden Baptiste had his first sickle cell crisis at age 2. Through elementary school, he ...