Gene therapy restores hearing in deaf mice
More than 70 different genes are known to cause deafness when mutated. Jeffrey Holt, PhD, envisions a day when patients with hearing loss have their genome sequenced and their hearing restored by gene therapy. A proof-of-principle study published today by the journal Science Translational Medicine takes a clear step in that direction, restoring hearing in deaf mice.
“Our gene therapy protocol is not yet ready for clinical trials—we need to tweak it a bit more—but in the not-too-distant future we think it could be developed for therapeutic use in humans,” says Holt, a scientist in the F.M. Kirby Neurobiology Center at Boston Children’s Hospital and an associate professor of Otolaryngology at Harvard Medical School.
Holt, with first author Charles Askew and colleagues at École Polytechnique Fédérale de Lausanne in Switzerland, focused on deafness caused by a gene called TMC1. Not only does TMC1 account for 4 to 8 percent of genetic deafness, it also encodes a protein that’s critical for hearing—helping to convert sound into electrical signals that travel to the brain.
To deliver the functioning TMC1 gene into the ear, the team inserted into an engineered virus called adeno-associated virus 1, or AAV1. For good measure, they added a promoter—a genetic sequence that turns the gene on only in certain sensory cells in the cochlea, known as hair cells.
They then injected the engineered AAV1 into the inner ears of mutant, deaf mice modeling the more common recessive form of TMC1 deafness, which causes profound hearing loss in children from a very young age, usually by around 2 years. After the injection, the animals’ sensory hair cells began responding to sound—producing a measurable electrical current—and activity began showing up in the auditory portion of their brainstems.
But most importantly, the mice began to hear—as evidenced when they were exposed to abrupt, loud tones in a “startle box.” When the mice jumped—indicating they heard the sound—a plate under their feet picked up the movement. After gene therapy, the mice began measurably startling at sounds beginning around 80 decibels. “Mice with TMC1 mutations will just sit there, but with gene therapy, they jump as high as a normal mouse,” says Holt.
Margaret Kenna, MD, MPH, a specialist in genetic hearing loss at Boston Children’s, is encouraged by the work. “Anything that could stabilize or improve native hearing at an early age is really exciting and would give a huge boost to a child’s ability to learn and use spoken language,” she says. “Cochlear implants are great, but your own hearing is better in terms of range of frequencies, nuance for hearing voices, music and background noise, and figuring out which direction a sound is coming from.”
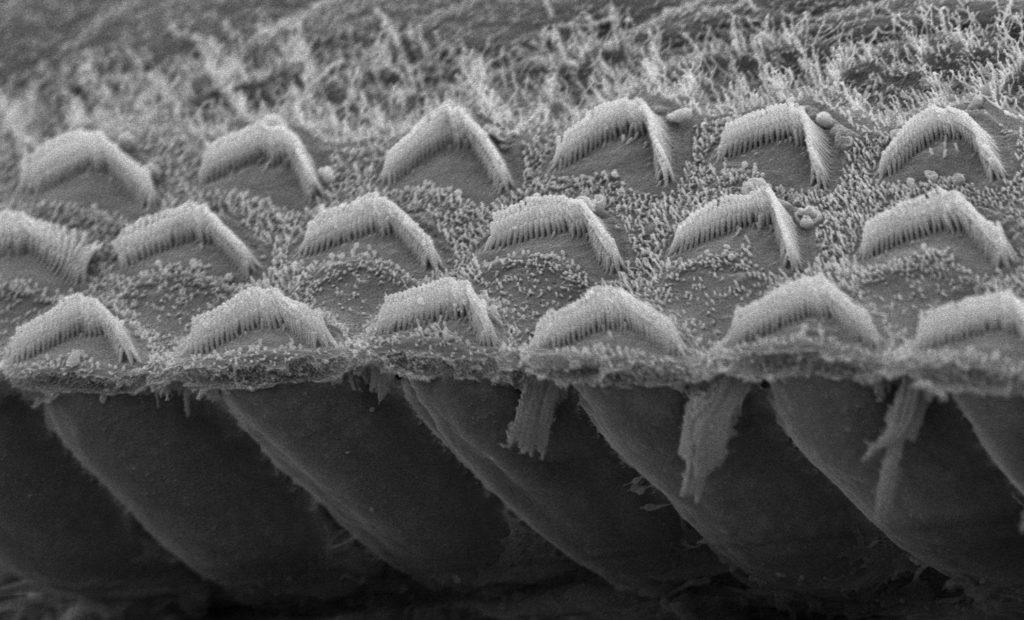
Sound transducers: How TMC works
Holt’s team showed in 2013 that TMC1 and the related protein TMC2 are critical for hearing, ending a rigorous 30-year search by scientists. Sensory hair cells contain tiny projections called microvilli, each tipped with a channel formed by TMC1 and TMC2 proteins. Arriving sound waves wiggle the microvilli, causing the channels to open. That allows calcium to enter the cell, generating an electrical signal that travels to the brain and ultimately translates to hearing.
Although the channel is made up of either TMC1 or TMC2, a mutation in the TMC1 gene is sufficient to cause deafness. However, Holt’s study also showed that gene therapy with the TMC2 gene could compensate for loss of a functional TMC1, restoring hearing in the recessive deafness model and partial hearing in a mouse model of dominant TMC1 deafness, in which patients gradually go deaf beginning around 10 to 15 years of age.
“This is a great example of how the basic science can lead to clinical therapies,” says Holt.
AAV1 is considered safe as a viral vector and is already in use in human gene therapy trials for blindness, heart disease, muscular dystrophy and other conditions. The researchers plan to further optimize their protocol and follow their treated mice to see if they retain hearing longer than the two months already observed.
Ultimately, Holt hopes to partner with Kenna and other clinicians to start clinical trials of TMC1 gene therapy within 5 to 10 years. He believes that other forms of genetic deafness may also respond to gene therapy.
Ernesto Bertarelli, co-chair of the Bertarelli Foundation, the primary funder of the research, is similarly optimistic: “These findings mark a defining moment in the way we understand, and can ultimately challenge, the burden of deafness in humans.”
Related Posts :
-

Mark’s winning pass with cochlear implants
Mark Bradshaw wanted to break out of his parents’ protective shell — as many teens do when they start pushing for ...
-

A sickle cell first: Base editing, a new form of gene therapy, leaves Branden feeling ‘more than fine’
Though he doesn’t remember it, Branden Baptiste had his first sickle cell crisis at age 2. Through elementary school, he ...
-

A universal gene therapy for Diamond-Blackfan anemia is poised for clinical testing
Diamond-Blackfan anemia (DBA), first described at Boston Children’s Hospital in 1938, is a rare blood disorder in which the bone ...
-

Cerebral adrenoleukodystrophy: How the Cooksons dodged a devastating disease
Heather Cookson believes that if she hadn’t insisted her son Ricky get a brain MRI to investigate his frequent ...