A new anti-cancer strategy: Overriding tumor edits
Cancers are clever and often find ways to dodge people’s immune systems, making them hard to eradicate. Immunotherapies such as CAR-T cells and checkpoint inhibitors can sharpen the immune system’s attack and cure the cancer. But they don’t work for most solid tumors.
We now know that tumors can edit their genes to evade immune control. A new study, for the first time, comprehensively surveyed which genes tumors are editing, providing a roadmap for designing better immunotherapies.
“No one had done an unbiased study of this before, because by the time a tumor becomes detected it has already finished gene editing,” says Judy Lieberman, MD, PhD, in the Program in Cellular and Molecular Medicine (PCMM) at Boston Children’s Hospital.
The findings, published in Nature Immunology, indicate that tumors edit multiple genes to avoid recognition by the immune system. But they also show a way to override these edits — curbing tumor growth in a human-like mouse model of breast cancer, which is notoriously resistant to immunotherapy.
Lieberman and Winston Hide, PhD, of Beth Israel Deaconess Medical Center, led the study. Ying Zhang, PhD, of PCMM (now at Peking University) and Pourya Naderi Yeganeh, PhD, of Beth Israel were the paper’s first authors.
Taking stock of cancer edits and fighting back
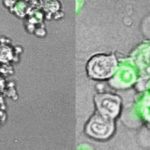
To capture how cancers edit genes, the researchers tracked breast tumors from their very origins. After turning on the Her2 oncogene in their mouse model, they used genome-wide single-cell RNA sequencing to identify which genes the newly-formed tumor was editing. They did this twice — one week and one month after Her2 oncogene induction.
Many of the genes the tumors edited are involved in the innate immune response — most notably genes that are stimulated by interferon and that enable immune cells to recognize tumor “danger signals.” The tumors silenced these genes epigenetically, primarily by chemically modifying the genes’ promoter sequences through methylation, effectively repressing immune activity.
Notably, the tumors edited relatively few genes associated with enhanced proliferation. “While the tumor also mutates to become more malignant, the dominant changes were immune,” says Lieberman.
To reverse tumor editing, she and her colleagues used an existing FDA-approved methylation inhibitor, decitabine. At low doses, decitabine reactivated the immune response, restoring multiple facets of immune control over the tumor. The end result: reduced tumor growth.
“There was a strong overlap between the genes the tumor edited early on and the genes awakened by the drug,” Lieberman notes.
The researchers saw an increase in T cells, natural killer cells, and dendritic cells and a sharp reduction in immunosuppressive myeloid-derived cells. Studying implanted breast and melanoma tumors, they found that decitabine restored function of genes involved in the innate immune system’s danger signaling pathways. These included pathways involving interferons or an inflammatory cell-death pathway known as pyroptosis.
More to explore
The work grew out of the Lieberman lab’s interest in gasdermin E, a potent tumor suppressor gene that triggers pyroptosis and that cancers repress through methylation. In 2020, Lieberman’s team showed that 20 of the 22 cancer-associated mutations they tested reduced gasdermin E function. When they expressed gasdermin E, anti-tumor immunity revved back into action, causing cancer cells to undergo pyroptosis.
Though the current work involved a breast cancer model, Lieberman believes that gene editing is a common feature of all cancers. Her lab plans to further validate its results and test decitabine, already used in some leukemias, in other solid tumors such as lung and ovarian cancer.
Lieberman also is interested in studying patients with genetic predispositions to cancer, who get screened frequently, to catch tumors in their earliest stages. “We hope to get tumor tissue from those patients and do what we did in the mice,” she says.
“More editing may occur when the tumors metastasize,” she adds. “That’s something else we want to study.”
Learn more about research in the Program in Cellular and Molecular Medicine (PCMM) and the Lieberman lab.
Related Posts :
-

Gasdermin E: A new approach to cancer immunotherapy
Tumors have figured out various ways to prevent the immune system from attacking them. Medicine, for its part, has fought ...
-

Exposing a tumor's antigens to enhance immunotherapy
Successful immunotherapy for cancer involves activating a person’s own T cells to attack the tumor. But some tumors have ...
-

Making immunotherapy safe for AML
Acute myeloid leukemia (AML), the second most common leukemia in children, is hard to treat and has a five-year survival ...
-

An unexpected journey reveals a potent way to attack tumors
Research on the effects of prenatal exposure to the Zika virus has yielded an unexpected dividend: a potentially promising way ...