Two drugs join forces against COVID-19

Two drugs, including one developed by a researcher at Boston Children’s Hospital, inhibit the SARS-CoV-2 virus that causes COVID-19 in tests of human cells. Both drugs, vacuolin-1 and apilimod, originally developed years ago, target a large enzyme called PIKfyve kinase. Prior to this study, little was known about this enzyme’s role in COVID-19 infection. Details of the discovery are published in a paper in PNAS.
· Studies with existing drugs vacuolin-1 and apilimod show they are highly effective at preventing SARS-CoV-2 infection in human cells.
· Both drug target the PIKfyve kinase enzyme, representing a potential new target for COVID-19 therapies.

“Our findings show that targeting this kinase through a small-molecule antiviral against SARS-CoV-2 may be an effective strategy to lessen the progression or seriousness of COVID-19,” says author Tomas Kirchhausen, PhD, of the Program in Cellular and Molecular Medicine at Boston Children’s, who discovered vacuolin-1. Apilimod was not developed by Kirchhausen, but by a company called LAM Therapeutics.
Earlier studies showed efficacy against Ebola
When Kirchhausen discovered vacuolin-1 16 years ago, he published a paper describing what it does in a variety of cell types. Several years later, Kirchhausen began a long collaboration with colleagues at Harvard Medical School in a Center for Excellence in Translational Research (CETR) focused on small molecules against emerging viruses. They showed that vacuolin-1 and apilimod, which have a similar chemistry, were both effective inhibitors against the Ebola virus. They did not publish their results at the time.
Both drugs prevent SARS-CoV-2 infection in cells
When COVID-19 began to hit the U.S. hard in early March, Kirchhausen’ s lab at Boston Children’s shut down like most others in the country. Before turning out the lights for good, he remembered vacuolin-1’s efficacy against Ebola and that the kinetics of cell entry of Ebola virus and coronaviruses like SARS-CoV-2 were similar.

Kirchhausen reached out to Sean Whelan, PhD, who was part of the CETR team at HMS but had since moved to Washington University. Together, the duo performed cell biology studies with SARS-CoV-2 virus in Whelan’s lab at Washington University.
“Within a week, we knew apilimod worked extremely well in preventing SARS-CoV-2 infection in human cells in the lab,” says Kirchhausen who first/initially published this discovery on the BioRxiv pre-print website in April 2020.
That paper also included a review of apilimod’s effectiveness against Ebola and SARS-CoV-2. “We found that like apilimod, Vacuolin-1 is a very strong inhibitor for viral infection in the lab,” adds Kirchhausen.
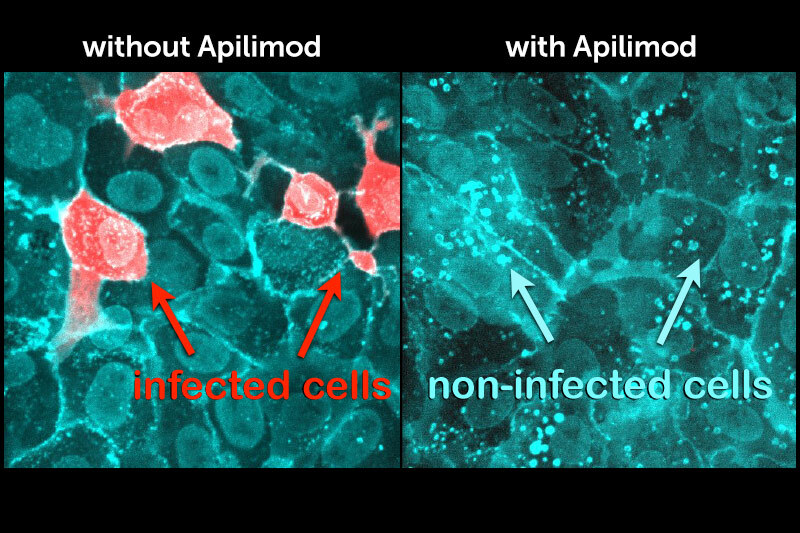
In an unexpected coincidence, an unrelated group posted a related paper. In a screen of 12,000 clinical-stage or FDA-approved small molecules, the researchers found that apilimod was one of the best drugs inhibiting SARS-CoV-2 virus replication. That paper has since been published in Nature.
Vacuolin-1 and apilimod join forces against COVID-19
Meanwhile, apilimod’s parallel development ultimately landed with AI Therapeutics after it failed to show any benefit in phase I and II clinical trials for treatment of autoimmune conditions, its original purpose. Although those trials were not successful, apilimod’s clinical testing in 700 healthy volunteers and patients showed it did not produce significant side effects even when given to patients for more than a year at high doses.
After Kirchhausen’s paper appeared on BioRxiv in April, AI Therapeutics and Kirchhausen combined efforts against COVID-19. Using some of the data from Kirchhausen’ s paper as well as information from drug screens by others, AI Therapeutics received FDA approval to study apilimod against COVID-19 to see if it reduces the seriousness of the disease.
Apilimod now in COVID-19 trial
In late July, AI Therapeutics announced a new randomized, double-blind, placebo-controlled study with apilimod (known as LAM-002 in the study). The study will test apilimod’s safety, tolerability, and efficacy in reducing the amount of virus in about 142 patients with confirmed early onset COVID-19 disease.
Looking forward, Kirchhausen hopes to identify other drugs to be given in addition to a PIKfyve kinase inhibitor. “Maybe an anti-inflammatory, or other drugs that target the proteases that activate the virus for cell entry, in addition to something that bring down viral load, like our drug,” he says.
Sean Whelan from Washington University is co-corresponding author on the paper. Yuan-Lin Kang and Yi-ying Chou from Boston Children’s are co-authors. Also included are Paul W. Rothlauf, Zhuoming Liu, James Brett Case, Rita Chen, and Michael Diamond from Washington University; and Timothy K. Soh and David Cureton from Harvard Medical School.
Read more COVID-19 research at Boston Children’s
Related Posts :
-

AI-enabled medical devices are burgeoning, but many haven’t been tested in children
Medical devices that incorporate artificial intelligence and machine learning are proliferating. In 2013, the FDA approved fewer than 10 such devices; by 2023, ...
-

Model enables study of age-specific responses to COVID mRNA vaccines in a dish
mRNA vaccines clearly saved lives during the COVID-19 pandemic, but several studies suggest that older people had a somewhat reduced ...
-

A sickle cell first: Base editing, a new form of gene therapy, leaves Branden feeling ‘more than fine’
Though he doesn’t remember it, Branden Baptiste had his first sickle cell crisis at age 2. Through elementary school, he ...
-

Will early intervention prevent asthma in school-age children?
Asthma affects about 1 in 10 children, often sending them to the emergency room or causing them to miss school. Allergic conditions ...