Parvalbumin neurons—new insight into the workings of a superhero brain cell
Say you’re a scientist in a movie, and you want to find out what gives a superhero his powers. You’d investigate any special suits he wears, whether he drinks any potions and what they are, right? Real-life scientists are following the same strategy to understand a powerful group of specialized brain cells.
Parvalbumin cells (PV-cells) are a population of inhibitory neurons found throughout the cerebral cortex. While small in number and size, they have the impressive capability to synchronize the electrical activities of other brain cells and orchestrate the timing of critical periods, interludes when the brain is more “plastic” and amenable to rewiring. Abnormalities in these pivotal cells are believed to make plasticity go awry, playing an important role in autism, schizophrenia and other neurodevelopmental disorders.
“The PV-cell is vulnerable in many mental illnesses,” says Takao K. Hensch, PhD, of the F.M. Kirby Neurobiology Center at Boston Children’s Hospital and professor of molecular and cellular biology and neurology at Harvard University. “So if we can find a way to maintain its health and well-being, then we might have a way to treat neurodevelopmental disorders, even later in life.”
Two key brain structures—one on the scene and one distant—shield and empower these superhero cells. New research from Hensch and collaborators illuminates their roles.
Perineuronal nets—shields against oxidative stress
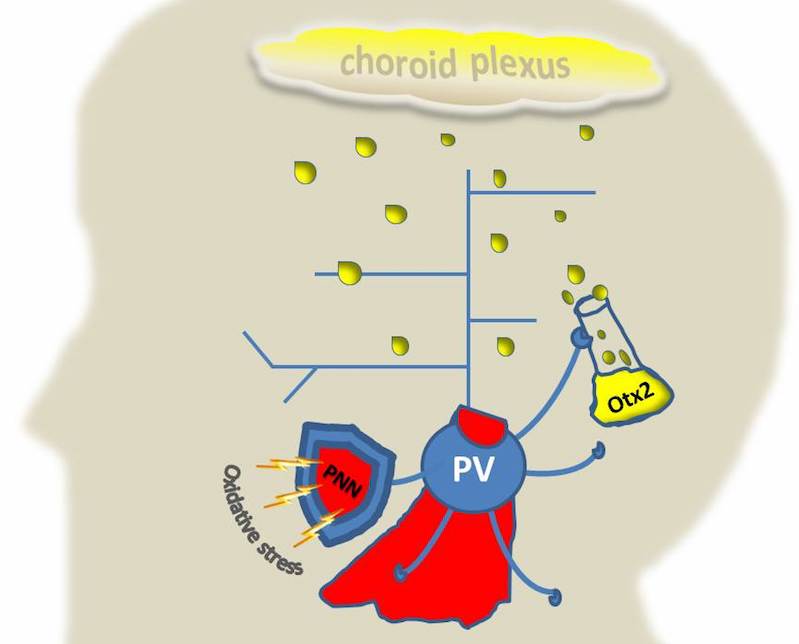
The high metabolic demands of hard-working PV-cells make them particularly vulnerable to oxidative stress, an inability to repair cell damage caused by toxins. This biochemical problem is thought to be central to many illnesses, including psychiatric disorders. A study published May 13 in the Proceedings of the National Academy of Sciences and led by Kim Q. Do, PhD, from the University of Lausanne in Switzerland in collaboration with Hensch, identified one protector against oxidative stress: sugar-rich structures known as perineuronal nets (PNNs) that enwrap PV-cell bodies as they mature.
Focusing on PV-cells in the prefrontal cortex of mice, Do, Hensch and co-first authors Jan-Harry Cabungcal, PhD, and Pascal Steullet, PhD, of the University of Lausanne, examined the impact of genetic impairments and environmental insults that trigger oxidative stress. Immature PV-cells lacking PNNs or clad with poorly condensed PNNs were highly susceptible to oxidative stress. Mature PV-cells, suited with highly condensed PNNs, were much less affected, but dissolving their PNNs made them behave more like their immature counterparts.
Interestingly, the PNNs themselves were susceptible to excessive oxidative stress. This result dovetails with earlier findings from the Hensch team suggesting that there is a positive feedback loop between PV-cells and PNNs, such that PV-cells help maintain the very outfit that safeguards them.
Choroid plexus—the unexpected source of a plasticity potion
Not only do the PNNs serve as shields for PV-cells, but they also serve up a potion that gives our superheroes a special power. As young PV-cells become clothed by PNNs, they start to amass a protein called Otx2, which has the power to initiate or turn off critical periods—those times in development when environmental experiences have the most impact on brain circuits.
Hensch’s team had discovered the plasticity powers of Otx2 in 2008, while studying the visual system in collaboration with Alain Prochiantz, PhD, of the Collège de France. The most surprising part of their findings was that at least some Otx2 comes from the retina. In essence, says Hensch, “the eye is telling the brain when to become plastic, rather than the brain developing on its own clock.” The PNNs, through their sugar residues, enable PV-cells to capture and accumulate Otx2.
Besides the retina, the collaborators found another surprising source of Otx2: the choroid plexus, a membranous structure lining the ventricles of the brain, forming part of the blood-brain barrier, and essentially converting blood from brain capillaries into the clear cerebrospinal fluid that bathes the entire cortex.
In a study published online June 13 in Cell Reports, Hensch, Prochiantz and co-first authors Henry H.C. Lee, PhD, of Boston Children’s Hospital and Julien Spatazza, PhD, now at the University of California, San Francisco, reveal that interfering with choroid plexus Otx2 production in adult mice diminishes Otx2 levels in PV-cells in the visual cortex. This restores a juvenile-like state of plasticity in the visual system, powerful enough to allow correction of amblyopia—a visual impairment that occurs when the brain, lacking strong visual input from one eye, rewires and becomes “blind” to that eye.
This is the first time the choroid plexus has been linked to brain plasticity. Since it can deliver Otx2 widely across the brain via the cerebrospinal fluid, these findings could lead to new thinking about therapies for disorders beyond the visual system where there may be aberrant cortical plasticity, like autism or schizophrenia.
“This offers an exciting opportunity, because the choroid plexus is a potentially accessible part of the brain,” says Hensch, who also directs Harvard’s Conte Center, a basic research group focused on developmental origins of mental illness. “It leads one to hope that we might be able to deliver agents through the bloodstream that adjust Otx2 levels in the choroid plexus and promote brain plasticity.”
Critical periods and PV-cell maturation occur at different times for different cortical regions, Hensch adds. If researchers can determine how and when Otx2 arrives in the “superhero” cells of various brain regions, it may be possible to better time interventions for different neurodevelopmental disorders.
Citations:
Cabungcal JH, Steullet P, Morishita H, Kraftsik R, Cuenod M, Hensch TK, & Do KQ (2013). Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proceedings of the National Academy of Sciences of the United States of America, 110 (22), 9130-5 PMID: 23671099
Spatazza J, Lee HH, Di Nardo AA, Tibaldi L, Joliot A, Hensch TK, & Prochiantz A (2013). Choroid-Plexus-Derived Otx2 Homeoprotein Constrains Adult Cortical Plasticity. Cell reports PMID: 23770240
Related Posts :
-

The thalamus: A potential therapeutic target for neurodevelopmental disorders
Years ago, as a neurology resident, Chinfei Chen, MD, PhD, cared for a 20-year-old woman who had experienced a very ...
-

Could peripheral neuropathy be stopped before it starts?
An increase in high-fat, high-fructose foods in people’s diets has contributed to a dramatic increase in type 2 diabetes. This, ...
-

Mutations during prenatal development may contribute to schizophrenia
Schizophrenia is known to have a genetic component, and variants in 10 genes have been identified as markedly increasing schizophrenia risk. ...
-

Delving into the causes of attention deficits: Childhood adversity, lost sleep, and dopamine
New research on the effects of adversity in childhood ties together stress, sleep loss, and attention deficits later in life. ...