Nick: Overcoming his fears to make a difference for others

People decide to participate in a clinical trial for lots of reasons. For 14-year-old Nick Brown, it came down to wanting to make a difference in the world.
Diagnosed with autism at age 5, Nick has had a lifelong aversion to hospitals, and especially to needles. Yet, when his parents told him about a new clinical trial for a medication for kids with autism, he decided to give it a try.
“When he realized the importance of the study and understood what a difference it could make in the world, participating in the trial became an important goal for him,” says his mom, Charline.
A promising medication
The medication, called balovaptan, has already shown promising results in adults and has been given breakthrough designation by the Food and Drug Administration (FDA). When Nick decided to enroll in late 2017, the trial was enrolling kids ages 8 to 17. It has since opened to those ages 5 to 7.
“This is the first medication specifically targeting social communication skills in kids with autism,” says Kate Pawlowski, research manager for the study. “Treatments usually mediate symptoms, not the core issues of autism.”
Charline heard about the study on social media and started looking at where the research was taking place. “We had never participated in a clinical trial before, but we were interested in this study because it was happening at Boston Children’s Hospital, which has such a great reputation,” she says. “We were also comforted by the fact that the medication had already been tested in adults.”
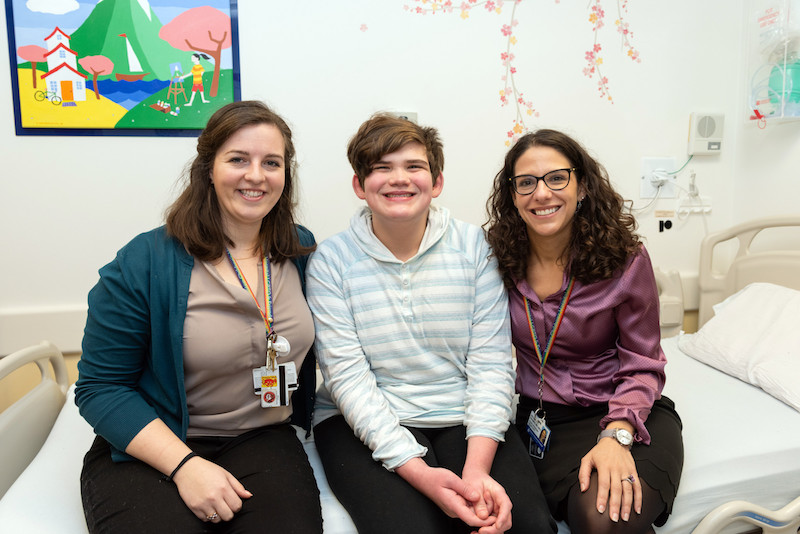
In December of 2017, Charline and Nick spoke with Kate on the phone, and she explained how the study worked and what was expected of Nick. “Kate was such a good fit from the start,” Charline says. “He had a lot of fears about the hospital and needles, but she explained everything to him in a way he could understand.”
Starting the trial
The first day of the trial was a little hard for Nick, but Charline says that with the help of Kate and the nurses, he made it through.
“I really appreciated the way they talked to him and helped him battle his anticipation and fear,” says Charline. For example, they gave him a choice about having anesthetic cream put on his arm before having his blood drawn, or using a J-tip, which is a device that delivers numbing medicine directly under the skin using compressed air.
“The J-tip was really great for Nick, I’ve never seen one used anywhere else,” says Charline. “The only difficult thing for him was the sound, so he wore headphones when they did it.”
As the study moved forward, Nick relaxed, and enjoyed being a part of it. He actually looked forward to the daylong hospital visits and had a really great rapport with the team. Even the blood draws became easier, and a visiting nurse was able to draw blood every two weeks without using the J-tip. “We made the trip to Boston every six weeks. Nick had multiple blood draws, EKGs and neurological exams, and yet he always looked forward to it.”
Changes in home and at school
Because the study was blinded, Nick, his family, and his doctors don’t know if he was actually taking the medication, or getting a placebo. But Charline says they started to notice changes in his behavior, both at home and in school.
“He missed 45 days of school in seventh grade because of social anxiety,” says Charline. “Now he’s in eighth grade and has missed fewer than ten days, and some of those were for Boston Children’s visits.”
Charline says that participating in the study has given Nick new confidence. At the end of seventh grade, he presented a project to his English class to educate people about autism, and then repeated the presentation for 40 first responders in the community. “I don’t think he would have been able to do that if he hadn’t had the experience of participating in the trial at Boston Children’s,” she says.

At home, he’s a new kid, too. “It’s been so great for our family,” says Charline. “Before it was hard to talk with him or play a game with him, because he took everything as criticism and would have a tantrum. Now he’s more relaxed.”
As Nick says, “When I was younger, I used to feel like something was not right. Now I feel like I can be proud of it, and autism is just part of my life.”
The family has also seen many indirect benefits of participating in an autism study which they hadn’t anticipated. Nick is better able to advocate for his needs to other people. Learning more about his diagnosis during the study has empowered him to speak up for himself and others who have autism. “When he was young, he’d say, ‘I have autism, I can’t do that,’” says Charline. “Now he says, ‘I have autism, let me explain what that means.’”
Continuing care in Boston
Now that Nick has completed the clinical trial, he is part of the open label study, officially taking the medication, and he continues to make improvements. “I told Kate that it doesn’t really matter if he was taking the medication during the trial or not. The experience of participating has changed his life and our lives for the better — it’s like night and day. We weren’t sure if a clinical trial would be too much for him, but we’re 100 percent certain we made the right decision.”
Although traveling to Boston Children’s was a bit of a hike for Nick and his family, who live more than an hour away in New Hampshire, Charline says the frequent trips were worth it. In fact, they have decided to continue Nick’s care at the Autism Spectrum Center at Boston Children’s.
“People ask why travel all the way to Boston,” says Charline. “And I can honestly say that the level of care we’ve received is unmatched. We have never had anything less than a stellar experience with every person we’ve encountered at Boston Children’s, from the cleaning crew and cafeteria workers to the clinical staff. We are big fans.”
Learn more about the Autism Spectrum Center.
Related Posts :
-

AI-enabled medical devices are burgeoning, but many haven’t been tested in children
Medical devices that incorporate artificial intelligence and machine learning are proliferating. In 2013, the FDA approved fewer than 10 such devices; by 2023, ...
-

A sickle cell first: Base editing, a new form of gene therapy, leaves Branden feeling ‘more than fine’
Though he doesn’t remember it, Branden Baptiste had his first sickle cell crisis at age 2. Through elementary school, he ...
-

Will early intervention prevent asthma in school-age children?
Asthma affects about 1 in 10 children, often sending them to the emergency room or causing them to miss school. Allergic conditions ...
-

First-of-its-kind clinical trial aims to improve outcomes in pediatric transplant patients
For the last 20 years, pediatric kidney transplant patients have been treated with the same immunosuppressive medication combinations and have been ...