Entry door for deadly C. difficile toxin suggests new mode of protection
Clostridium difficile, also called “C. diff,” tops the CDC’s list of urgent drug-resistant threats. Marked by severe diarrhea and intestinal inflammation, C. diff has become a leading cause of death from gastrointestinal illness, causing half a million infections a year in the U.S. alone.
C. diff flourishes best in hospitals and long-term care facilities where people are on long-term antibiotic treatment. “Antibiotics clear out the normal intestinal bacteria and create a space for C. diff to colonize and grow in the colon,” says Min Dong, PhD, who researches bacterial toxins in the Department of Urology at Boston Children’s Hospital.
In today’s Nature, Dong and postdoctoral fellow Liang Tao, PhD, together with researchers at University of Massachusetts Medical School, reveal how C. diff’s most potent toxin gets into cells. The toxin’s entryway, a receptor called Frizzled, provides an important and interesting clue to fighting the hard-to-eradicate infection.
Identifying the Frizzled receptor
C. diff produces a variety of toxins that target and disrupt the epithelial cells lining the colon. Toxins A and B are the best known. Dong chose to focus on toxin B since it’s been shown to cause disease on its own, even when toxin A is absent.
“We knew that these toxins may hijack a cell surface protein as its landing pad or receptor,” Dong says. “Yet the specific receptor for toxin B had remained elusive.”
Tao and Paul Meraner, MD, a fellow in the lab of Abraham Brass, MD, PhD, at UMass Medical School, identified Frizzled through a novel approach that leveraged CRISPR/CAS9 gene editing technology.
“CAS9 technology originates from bacteria, so it was gratifying to use CAS9 to learn about one of the worst types of bacteria in the hospital,” says Brass. “As a GI doctor, I’ve seen too much hardship caused by C. diff and look forward to the day when we beat it.”
A protective gene deletion
Together, applying CRISPR/CAS9 across the genome, Tao and Meraner systematically mutated genes one by one in cultured human cells. They now had plates of cells, each cell with a mutation disabling a different gene. “We then added toxin B to these plates and looked for cells that were resistant,” says Tao.
Most cells were killed by the toxin, but not all. After four rounds of screening, Tao and Meraner had a population of cells that were unharmed. Those, they reasoned, had a mutation that protected them from the toxin. But in which gene?
The researchers turned to next-generation sequencing, which revealed a number of mutations in the surviving cells. Among them was Frizzled, a cell surface protein. Tao went on to show that toxin B indeed uses Frizzled as its receptor to invade cultured human cells.
Tao next teamed up with Ji Miao, PhD and David Breault, MD, PhD, in Boston Children’s Division of Endocrinology, who study colonic stem cells. Together, they used colonic stem cells to create “organoids” — 3-D cultures of intestinal tissue in miniature. When the gene for Frizzled was deleted, the organoids withstood exposure to the toxin. In contrast, organoids that retained the Frizzled gene showed obvious damage.
Finally, Tao worked with Jie Zhang, PhD, a senior scientist in the Dong lab, to do studies in mice, which gave parallel results.
Potential C. diff therapeutic strategies
Interestingly, Frizzled is also known be the entryway for signals that help keep the colon healthy. It receives signals critical to repairing and maintaining tissue in the colon, via a well-known pathway known as Wnt.
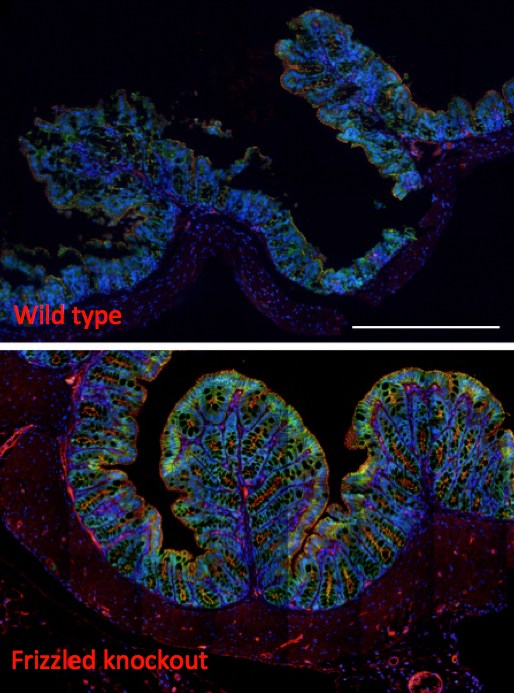
Protection from C. diff?
Knocking out the Frizzled receptor in a mouse model (bottom panel) prevents C. diff toxin B from getting into the colon tissues, which remain mostly intact. Dong believes it’s possible to get the same results with a drug. In wild type mice, still susceptible to the toxin, the colon’s epithelial layer is clearly damaged (shown by loss of molecular markers of cell-cell junction).
“Stem cells in the colon rely on Wnt signaling to keep them functioning and generating new cells,” explains Dong. “Toxin B and Wnt signals compete to bind to the Frizzled receptors. This suggests that toxin B may directly inhibit Wnt signaling in cells.”
To confirm this, Tao and Dong worked with Wnt experts Xinjun Zhang, PhD, and Xi He, PhD, at Boston Children’s F.M. Kirby Neurobiology Center. Together, they demonstrated that toxin B strongly inhibits Wnt signaling.
“This finding suggested that toxin B can be particularly detrimental to colonic stem cells,” says Dong. “It would be important to restore Wnt activity and maintain the health of stem cells in combating these toxins.”
The findings also suggest potential ways of protecting the colon. Tao and Jie Zhang found that a recombinant fragment of Frizzled protein can “soak up” toxin B. Another potential approach would be to stimulate Wnt signaling directly, says Dong.
Cancer treatment?
The Wnt pathway is also known to be important in cancer cells. Pharmaceutical companies are trying to develop antibodies that would block Frizzled. Dong and colleagues speculate that a fragment of the C. diff toxin — one that isn’t toxic — could be used to study Wnt signaling or even possibly as a cancer therapy.
Liang Tao was first author on the paper. The study was supported by the NIH, the Bill and Melinda Gates Foundation, the Timothy Murphy Fund, the Harvard Digestive Diseases Center, the Boston Children’s Hospital Intellectual and Developmental Disabilities Research Center and the American Cancer Society. Both Dong and Brass are recipients of the Investigators in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Related Posts :
-

Team spirit: How working with an allergy psychologist got Amber back to cheering
A bubbly high schooler with lots of friends and a passion for competitive cheerleading: On the surface, Amber’s life ...
-

Thanks to Carter and his family, people are talking about spastic paraplegia
Nine-year-old Carter may be the most devoted — and popular — sports fan in his Connecticut town. “He loves all sports,” ...
-

No limitations: How Flora found answers for MOG antibody disease
Flora Ringler’s fifth birthday didn’t turn out as she had hoped. She and her family were vacationing in ...
-

Genetic causes of congenital diarrhea and enteropathy come into focus
Congenital diarrheas and enteropathies are rare and devastating for infants and children. Treatments have consisted mainly of fluid and nutritional ...





