Diet, the microbiome, and how insulin resistance causes metabolic syndrome

Up to a third of U.S. adults have metabolic syndrome, a constellation of high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels. Now considered to be epidemic, metabolic syndrome increases people’s risk of diabetes, heart disease, and stroke, as well as kidney and neurodegenerative disease.
Metabolic syndrome is closely associated with insulin resistance, in which cells fail to respond appropriately to insulin, a hormone that regulates multiple cell metabolic processes such as taking up sugar from the blood. But thus far, scientists have been unable to understand how insulin resistance might produce the different features of metabolic syndrome.
Finally, a new study puts the pieces together. It involves a surprise pairing of two infamous molecules, with diet and gut microbes playing supporting roles.
The TMAO of metabolic syndrome, with a PERK
Over past five years, studies have found an increase in a compound called TMAO in people with insulin resistance, diabetes, kidney disease, cardiovascular disease, and neurodegenerative disease. Mice fed TMAO have been found to develop glucose intolerance, thrombosis, kidney disease and neurodegenerative disease.

“When you reduce TMAO in a mouse model, it has a lot of benefits – it reduces blood glucose levels, and decreases atherosclerosis and dyslipidemia,” says Sudha Biddinger, MD, PhD, of Boston Children’s Hospital’s Division of Endocrinology. “TMAO seemed important physiologically, but its mechanism of action was a real mystery. We wanted to know how this molecule works.”
The study, published September 19 in Cell Metabolism, identified, for the first time, what cellular receptor TMAO uses to exert its effects: a molecule called PERK. PERK is a linchpin of stress signaling within cells – specifically, stress on the endoplasmic reticulum (ER), a part of the cell where proteins are assembled, folded, and dispatched to do their jobs. Under conditions of ER stress, misfolded proteins accumulate and PERK sends distress signals that can ultimately trigger cell death pathways.
“Anything that produces ER stress converges on this PERK molecule,” says Biddinger, the study’s senior author.
Cells in distress
The interplay between TMAO and PERK could give a new framework for understanding insulin resistance and metabolic syndrome, and the host of diseases they predispose us to — stroke, heart attack, kidney failure and more, says Biddinger.
“Our work suggests that this could all be mediated by a TMAO/PERK pathway — tying in insulin resistance with all different components of metabolic syndrome. Now we can understand how they’re all fitting together,” she says. “It seems to be the missing piece of the puzzle.”
Microbes, diet, and metabolic syndrome
An exciting aspect of the study is that TMAO is made from a breakdown product of certain intestinal microbes, courtesy of an enzyme called FMO3. Knowing this allowed the researchers to reduce levels of TMAO in two different ways: by inhibiting FMO3, or by manipulating the gut microbiome to favor bacteria that don’t make TMAO. Both approaches reduced PERK activation in the livers of obese, insulin-resistant mice and also reduced hyperglycemia.
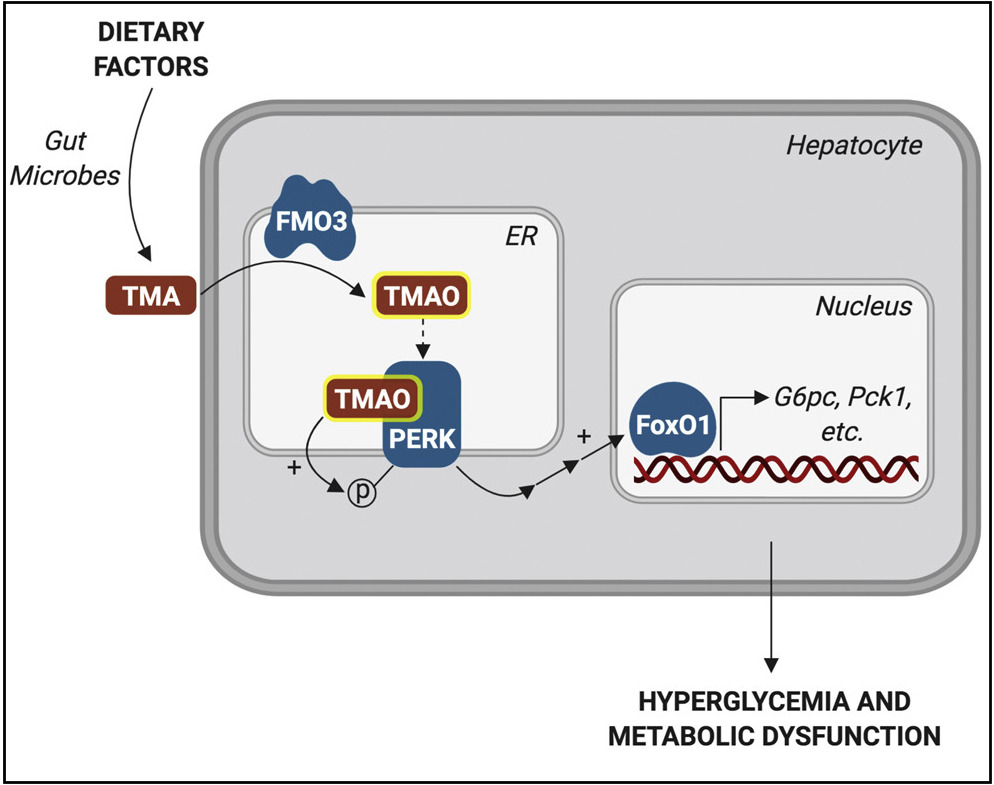
“Now we know very specific intestinal bugs that can talk to ER stress pathways in liver,” says Biddinger. “When we change one enzyme in the bug, it changes what the liver is saying.”
And here’s where dietary factors come in. The inhibitor used to suppress FMO3 (and, therefore, TMAO) is derived from cruciferous vegetables like cauliflower and brussels sprouts. In contrast, foods such as red meat and eggs are high in choline, which some gut microbes convert to TMAO.
Avoiding metabolic syndrome?
So does this mean we should eat more veggies and less red meat to reduce TMAO levels and improve our metabolic status? Biddinger is loath to make dietary declarations without more evidence, but is happy to get behind eating more vegetables. She notes that the two FMO3 inhibitors used in the study are commercially available as nutritional supplements.
“Our findings open the door to new treatments or dietary approaches that might reduce TMAO, decrease the effects of insulin resistance, and prevent metabolic syndrome and its health effects,” she says.
Learn more about the Division of Endocrinology.
Sifan Chen of Boston Children’s Division of Endocrinology was first author on the study. See the paper for a full list of authors. The work was funded by the National Institutes of Health (R01HL109650, R00DK100539), the American Heart Association, and the German Ministry of Education and Research (BMBF) and the State of Brandenburg (82DZD00302).
Related Posts :
-

New genetic insights could change how we treat, and talk about, polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) has long been viewed as a hormonal disorder affecting women of reproductive age. However, ongoing research ...
-

Partnering diet and intestinal microbes to protect against GI disease
Despite being an everyday necessity, nutrition is something of a black box. We know that many plant-based foods are good ...
-

Addressing food insecurity and nutrition challenges in pediatric type 1 diabetes care
Managing type 1 diabetes can be overwhelming for children and families. As children learn to live with the disease, many cut ...
-

A new tool could exponentially expand our understanding of bacteria
How do bacteria — harmless ones living in our bodies, or those that cause disease — organize their activities? A new study, ...