Drug ‘cocktail’ could restore vision in optic nerve injury
When Zhigang He, PhD, started a lab at Boston Children’s Hospital 15 years ago, he hoped to find a way to regenerate nerve fibers in people with spinal cord injury. As a proxy, he studied optic nerve injury, which causes blindness in glaucoma — a condition affecting more than four million Americans — and sometimes in head trauma.
By experimenting with different growth-promoting genes and blocking natural growth inhibitors, he was able to get optic nerve fibers, or axons, to grow to greater and greater lengths in mice. But what about vision? Could the animals see?
“The question was, is this regenerating axon functional?” says He, who is part of Boston Children’s Department of Neurology and F.M. Kirby Neurobiology Center.
Other teams, including the lab of Larry Benowitz, PhD, at Boston Children’s, have been able to achieve partial vision. But they’ve relied on genetic techniques that can only be done in a lab, and methods have involved deleting or blocking tumor suppressor genes, which encourages regeneration but could also promote cancer.
A study published today by Cell, led by He and Michela Fagiolini, PhD, demonstrates that vision can be restored using an approach that could realistically be applied in the clinic and does not interfere with tumor suppressor genes.
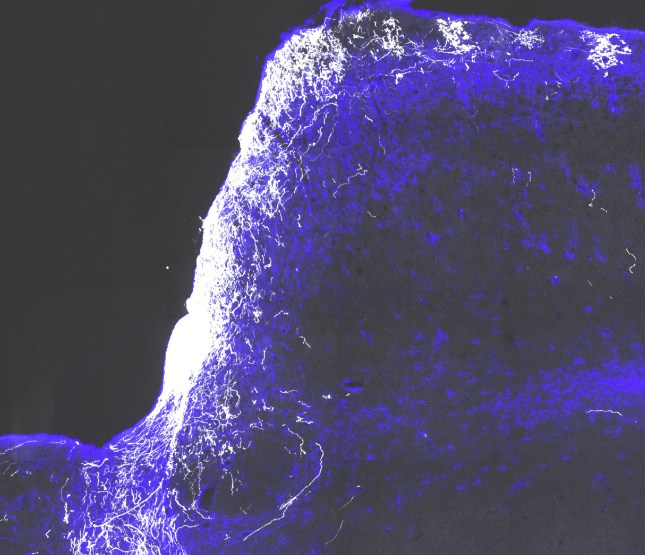
In optomotor tests, above, previously blind mice turned their heads to follow patterns of moving bars after given the treatment. “By making the bars thinner and thinner, we found that the animals could not only see, but they improved significantly in how well they could see,” says Fagiolini.
Getting nerves to conduct
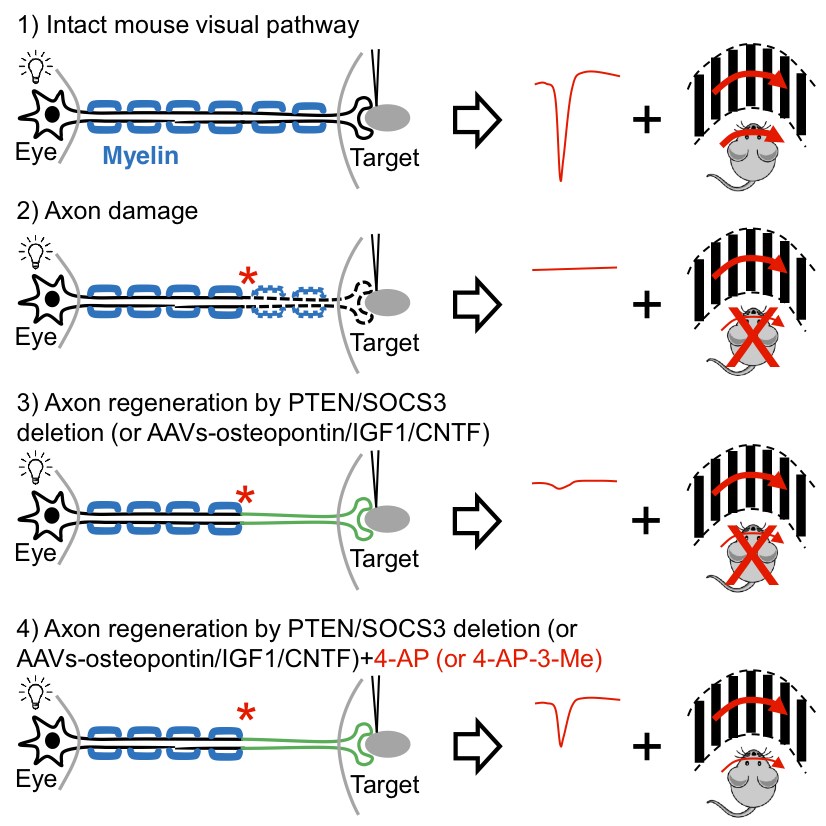
He, Fagiolini and colleagues started with gene therapy to deliver three key growth factors (osteopontin, insulin-like growth factor 1 and ciliary neurotrophic factor. This approach got axons to regenerate and even form connections, or synapses, with their target cells in the brain. But the axons weren’t able to carry signals all the way from the eye to the brain, because they lacked myelin, an insulating sheath that helps propagate nerve signals over long distances.
“We found that the regenerated axons are not myelinated and have very poor conduction — the travel speed is not high enough to support vision,” says He. “We needed some way to overcome this issue.”
Turning to the medical literature, they learned that a potassium channel blocker, 4-aminopyridine (4-AP), helps strengthen nerve signals when myelin is absent. The drug is marketed as AMPYRA for multiple sclerosis, which also involves a loss of myelin. When they added it, the signals were able to go the distance.

A paradigm for treating glaucoma and optic nerve injury
While the study used a gene therapy virus called AAV to deliver the growth factors, He and Fagiolini are testing whether injecting a “cocktail” of growth factor proteins directly into the eye could be equally effective.
“We’re trying to better understand the mechanisms and how often the proteins would have to be injected,” says He. “The gene therapy virus we used is approved for clinical study in eye disease, but a medication would be even better.”
With regeneration kick-started, 4-AP or a similar drug could then be given systemically to maintain nerve conduction. Because 4-AP has potential side effects including seizures if given chronically, He and Fagiolini have begun testing derivatives (not yet FDA-approved) that are potentially safer for long-term use.
And they’re further testing the mice to better understand the extent of visual recovery and whether their approach might get myelin to regrow over time.
“The drugs might need to be paired with visual training to facilitate recovery,” says Fagiolini. “But now we have a paradigm to push forward.”
Fengfeng Bei, PhD, and Hing Cheong (Henry) Lee, PhD, of the F.M. Kirby Neurobiology Center at Boston Children’s, were co-first authors on the paper. The study was supported by the National Eye Institute (NIH grant EY021526), the Miriam and Sheldon G. Adelson Medical Research Foundation and the Georgetown Center for Brain Plasticity and Recovery.
Related Posts :
-

Hope in sight for autosomal dominant optic atrophy (ADOA)
Autosomal dominant optic atrophy (ADOA), the most common genetic optic neuropathy, is an insidious disease. It often presents slowly during ...
-

New research sheds light on the genetic roots of amblyopia
For decades, amblyopia has been considered a disorder primarily caused by abnormal visual experiences early in life. But new research ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

The thalamus: A potential therapeutic target for neurodevelopmental disorders
Years ago, as a neurology resident, Chinfei Chen, MD, PhD, cared for a 20-year-old woman who had experienced a very ...