First-of-their-kind findings turn conventional wisdom about diffuse hemispheric glioma on its head

Diffuse hemispheric glioma, H3G34-mutant (DHG-H3G34) is a type of high-grade glioma that typically affects adolescents and young adults. The lack of targeted treatments contributes to a very poor prognosis for patients with these malignant brain tumors.
But what if some of what we thought we knew about DHG-H3G34 turned out to be wrong?
That’s the implication of a new study published in Cancer Cell and led by Mariella Filbin, MD, PhD, co-director of the Brain Tumor Center at Boston Children’s Hospital and Dana-Farber Cancer Institute. She and her colleagues recently found that the cellular makeup of DHG-H3G34 appears to differ from that of other gliomas — opening the door to potential treatment targets. Their study also provides the first patient data to support a targeted approach.
‘This must be a mistake’
DHG-H3G34 comprise about 15 to 20 percent of high-grade gliomas and have been shown to be molecularly distinct from other types of high-grade gliomas: Previous research suggested that DHG-H3G34 might originate from neuronal cells — those neurons that transmit signals within the brain. However, much remained unknown about the functional role of these neuronal cells and whether they represented an opportunity for treatment strategies.
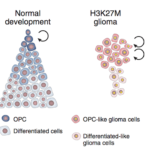
To learn more, Filbin and her colleagues used bulk and single-cell multi-omics, as well as spatial analyses, to characterize the full developmental spectrum of DHG-H3G34 interneuron-like tumor cells in 200 pediatric patient tumors. They found that — unlike other high-grade glioma subtypes that have undergone RNA sequencing — DHG-H3G34 tumor cells didn’t appear to derive from a common glial cell ancestor. On the contrary, tumor cells seemed to avoid the oligodendroglial lineage all together. Instead, they appeared to mirror spatial nests of early human interneuron development.
“At first, we thought this must be a mistake,” says Filbin. But the results held up with repeated testing. In fact, the cells resembled GABAergic neurons — nerve cells that produce the inhibitory neurotransmitter GABA.
Identifying treatment targets for DHG-H3G34
Although these findings alone would have transformed the way we view DHG-H3G34, Filbin and the team decided to push the envelope further.
“We wanted to determine whether this interneuronal developmental lineage might make DHG-H3G34 tumors vulnerable to targeted therapies,” she explains.
To that end, Filbin and team performed genomic-wide CRISPR Cas9 screens on DHG-H3G34 cell models and combined their data with that from their colleagues in the Institute of Cancer Research, London. They found that knocking out glial genes had little effect on tumor cells — but when genes expressed in early neuronal stem cells were knocked out — specifically SOX2 — tumor growth slowed. Further, both in vitro and in mouse models, when the gene CDK6 was knocked out, tumor cells either died or eventually developed into inter-neurons. Mice with DHG-H3G34 who were given a CDK4/6-inhibiting drug lived significantly longer but ultimately still died.
Drawing from these findings, the investigators identified and treated a patient who had progressive, multiply recurrent DHG-H3G34 with a CDK4/6-inhibitor. Although the patient’s tumor eventually worsened again, she did achieve 17 months of stable disease while on the drug. This is the first patient data to support this strategy in a patient with highly recurrent disease with this type of brain tumor.
Filbin hopes that these results mark the beginning of a sea change in the way DHG-H3G34 is understood and treated.
“Not only do our findings go against current knowledge that gliomas should come from and mimic glial cells only, but they also open up a whole new array of potential drug targets,” she says. “We’ve moved the needle, but we need to move it even more.”
Filbin is now designing a clinical trial for patients with DHG-H3G34, in collaboration with Karen Wright, MD, director of Pediatric Neuro-Oncology Experimental Therapeutics for the Dana-Farber/Boston Children’s Cancer and Blood Disorders Center.
Learn more about the Brain Tumor Center.
Related Posts :
-

Research opens a window into understanding deadly brain tumors
Formerly known as diffuse intrinsic pontine gliomas, diffuse midline gliomas (DMGs) are highly aggressive tumors found in the midline of ...
-

Solving the DIPG puzzle a single cell at a time
For more than 15 years, pediatric neuro-oncologist Mariella Filbin, MD, PhD, has been on a scientific crusade to understand DIPG (...
-

Single-cell sequencing reveals glioblastoma's shape-shifting nature
Glioblastoma, a cancer that arises in the brain’s supporting glial cells, is one of the worst diagnoses a child ...
-

Diving into the dark side of ependymoma
Mariella Filbin, MD, PhD, a neuro-oncologist at Dana-Farber/Boston Children’s Cancer and Blood Disorders Center, is driven by a ...