Designing a coronavirus vaccine for next year – and the years beyond
As the number of coronavirus infections swell daily across the globe, strategies for developing a safe and effective vaccine are rapidly moving forward. In response to this public health crisis, the Precision Vaccines Program (PVP) at Boston Children’s Hospital is on the front lines of developing a coronavirus vaccine targeted especially toward older populations, those who are at greatest risk of developing coronavirus disease 2019 (COVID-19), caused by severe acute respiratory syndrome-2 coronavirus (SARS-CoV-2).
According to the World Health Organization, older people, and people with pre-existing medical conditions appear to be more vulnerable to becoming severely ill with COVID-19.
“Elderly individuals have a different immune system than healthy middle aged adults and often do not respond as robustly to immunization, so a one- size vaccine does not fit all,” says Ofer Levy, MD, PhD, director of the PVP.
Focusing on adjuvants to boost immunity
The current antigen used for vaccine development (the part of the virus that the immune system “remembers”) is the coronavirus spike protein, named because it sits atop the spike of a coronavirus particle. Vaccine-induced antibodies, made by the immune system against the spike protein, can prevent infection.
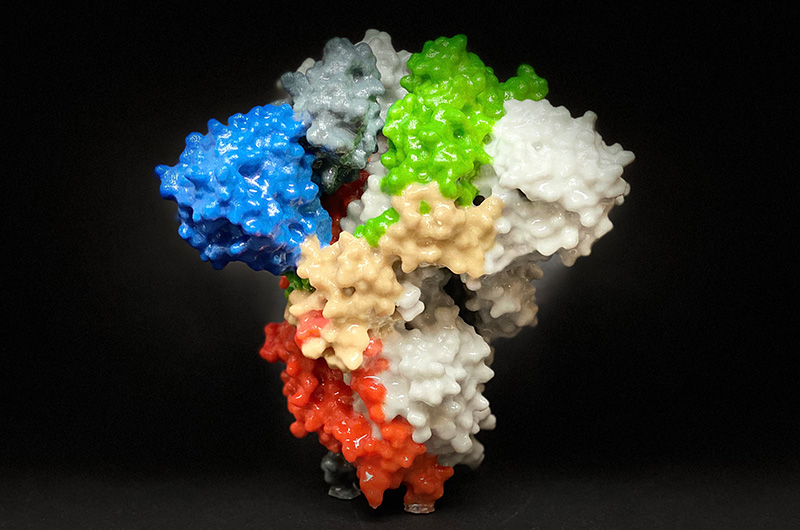
PVP’s strategy is to combine the coronavirus spike protein with known vaccine adjuvants or new ones discovered through the PVP’s Adjuvant Discovery Program. Adjuvants are small molecules added to a vaccine to boost the recipient’s immune response.
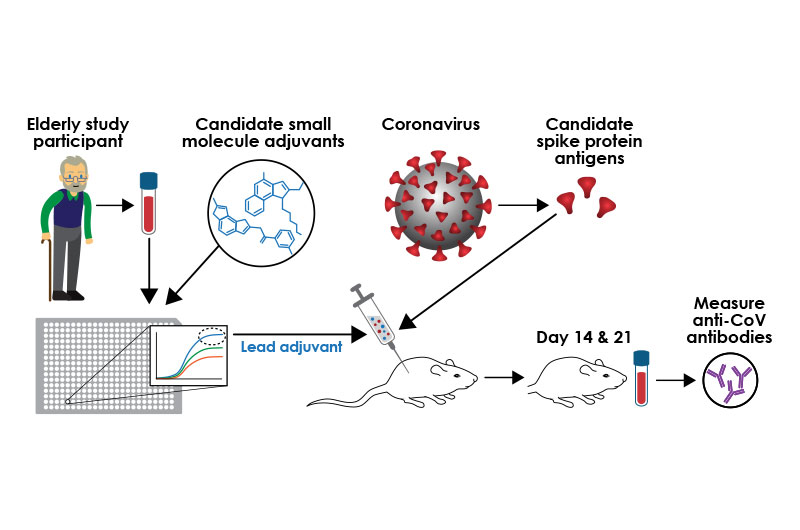
“Overall, we are hoping that a precision adjuvant approach will assist the various ongoing vaccine efforts across the globe,” says Levy. “Adjuvants can be crucial for getting a stronger, longer-lasting, broader immune response, especially among those with weakened immunity like the elderly,” says Levy.
The antigen is typically the most expensive part of a vaccine, Levy notes. “If we want to be able to provide billions of vaccine doses, adjuvants can be the answer as they enhance the immune system, so much less antigen is needed to get a protective immune response.”
The PVP plans on testing a variety of adjuvants and adjuvant combinations in human white blood cells sourced from older people. Researchers will then study the adjuvant-induced immune responses.
“Our screen, comparing individual and combination adjuvants with and without the coronavirus antigen, will identify an adjuvant combination that most effectively induces an optimal immune response in the elderly,” Levy says. These screens will start immediately and continue over the next six to eight weeks.
“We’re hoping to have a clear signal within the next few months which adjuvanted vaccine to go forward with in clinical testing,” Levy adds.
Testing in mice
The group is also studying coronavirus immune responses in a living animal model. The first mice have already been inoculated with a similar coronavirus spike protein derived from the SARS-2003 coronavirus with or without a lead adjuvant combination to get an early read on measuring an antibody response.

Several leading laboratories are providing essential input to the PVP in efforts to develop an effective, targeted coronavirus vaccine that works well for older individuals:
- Barney Graham, MD, PhD, deputy director of the Vaccine Research Center at the National Institute of Allergy and Infectious Diseases (NIAID), provided the coronavirus spike protein to the PVP a few weeks ago.
- Florian Krammer, PhD, at the Icahn School of Medicine at Mount Sinai, provided SARS-COV-2 virus from the current strain
- Peter Hotez, MD, PhD ,and Maria Bottazzi, PhD, at Baylor University, contributed antigen from the 2003 SARS outbreak.
- Lindsey Baden, MD, director of Clinical Research at the Brigham and Women’s Hospital, provided blood samples from elderly patients.
We’re hoping to have a clear signal within the next few months which adjuvanted vaccine to go forward with in clinical testing.” Ofer Levy
Envisioning a seasonal coronavirus vaccine
Looking ahead, the PVP is looking to create a vaccine platform and systematic program that would facilitate vaccine development in any future coronavirus outbreaks.
“Dr. Anthony Fauci, director of the NIAID, has warned of the potential of SARS-CoV-2 to become a seasonal virus like flu,” says David J. Dowling, PhD, of the Adjuvant Discovery and Development Laboratory within the PVP. “If so, the biomedical community may need to consider developing a multi-valent yearly seasonal vaccine effective against multiple coronaviruses.”
As ongoing international surveillance identifies circulating coronavirus strains, the PVP envisions collaborating with other laboratories to obtain corresponding viral spike proteins and modeling responses of the elderly to different adjuvant/antigen combinations to determine which is most effective.
Three COVID-19 vaccine concepts
Levy and Dowling estimate that more than 24 COVID-19 vaccine candidates are in development globally. These vaccines fall into three general types:
RNA-based vaccines. The first COVID-19 vaccines developed have been created using SARS-CoV-2 RNA. “This approach is innovative and attractive, but even if proven effective it may be difficult to create hundreds of millions of doses, and each dose may be relatively expensive as it may require a fair amount of RNA,” says Dowling. “While there is less experience with human testing for this approach, it is possible that adjuvants may help enhance the immune response when such vaccines are administered to older populations, and reduce the amount of RNA needed in each vaccine.”
DNA-based technology. Like RNA-based vaccines, this promising approach uses the genetic material of the virus to produce a vaccine. “You can make more vaccine doses with DNA than with RNA, but it is unclear whether the production could be rapidly scaled to meet the massive international demand,” says Dowling. “And, to our knowledge, these nucleic acid vaccines have not yet been tested in elderly patients either.”
Building on earlier coronavirus vaccines. This is the approach PVP is taking: learning from earlier vaccines from prior coronavirus outbreaks and making them more effective. Adds Dowling: “If you add an adjuvant chosen specifically for optimal activity in an older population, not only does it work better in that group, but it may dramatically reduce the cost per vaccine dose by reducing the amount of antigen required.”
Read more about COVID-19
Related Posts :
-

Model enables study of age-specific responses to COVID mRNA vaccines in a dish
mRNA vaccines clearly saved lives during the COVID-19 pandemic, but several studies suggest that older people had a somewhat reduced ...
-

Will people accept a fentanyl vaccine? Interviews draw thoughtful responses
In 2022, more than 100,000 people died from opioid overdoses in the U.S., according to the National Center for Health Statistics. ...
-

New insight into the effects of PPIs in children
Proton-pump inhibitors (PPIs) are frequently prescribed to suppress stomach acid in patients with gastroesophageal reflux disease (GERD). Prescribing rates of ...
-

Creating the next generation of mRNA vaccines
During the COVID-19 pandemic, mRNA vaccines came to the rescue, developed in record time and saving lives worldwide. Researchers in ...