How things work: Scientists find cellular channels vital for hearing
Ending a 30-year search by scientists, researchers have identified two proteins in the inner ear that are critical for hearing, which, when damaged by genetic mutations, cause a form of delayed, progressive hearing loss.
The proteins are essentially transducers: They form channels that convert mechanical sound waves entering the inner ear into electrical signals that talk to the brain. Corresponding channels for each of the other senses were identified years ago, but the sensory transduction channel for both hearing and the sense of balance had been unknown.
The channels are the product of two related genes known as TMC1 and TMC2. TMC1 mutations were first reported in people with a prominent form of hereditary deafness back in 2002 by Andrew Griffith, MD, PhD, of the National Institute on Deafness and Other Communication Disorders (NIDCD) and collaborators. Children with recessive mutations in TMC1 are completely deaf at birth.
A concurrent report described “Beethoven,” a model mouse that becomes deaf by the second month of life. Children that carry a similar TMC1 mutation become completely deaf by the age of 10 to 15 years.
Then, in 2011, the laboratory of Jeffrey Holt, PhD, in Boston Children’s Hospital’s the Department of Otolaryngology and F.M. Kirby Neurobiology Center, showed TMC2 to have a redundant function, with the ability to compensate, at least in part, if TMC1 is defective. That happens sometimes in biology and may explain why hearing loss occurs gradually after birth.

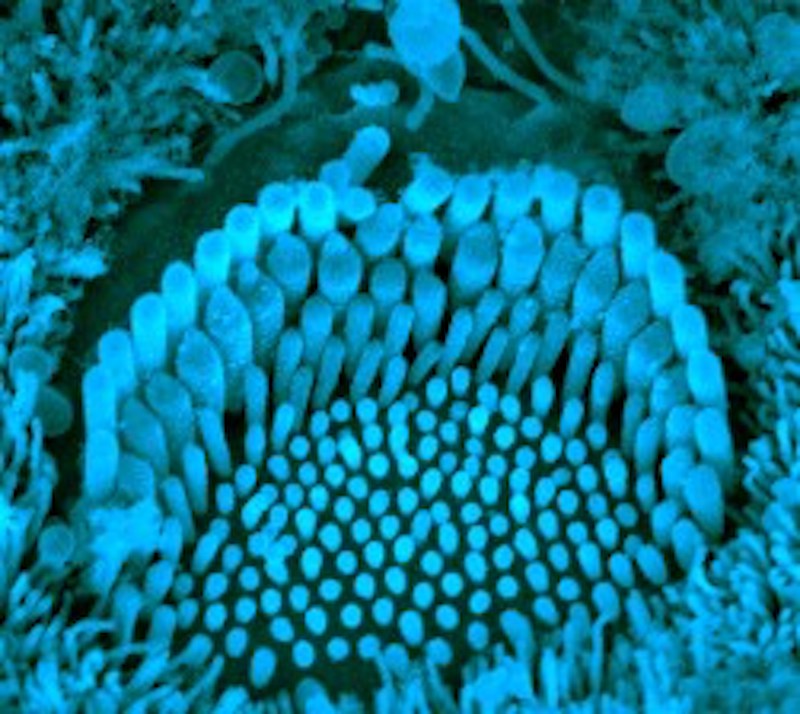
But until now, it wasn’t clear what the genes do or how they cause deafness. In the new work, just published in Neuron, Holt, Griffith, first author Bifeng Pan, PhD, of Boston Children’s and colleagues studied sensory hair cells from the cochleas of the Beethoven mice, and showed that the TMC1 and TMC2 proteins form ion channels necessary to get calcium into the cells. When TMC1 was mutated, this calcium influx was reduced, and the resulting electrical current was weaker in response to sound.
“This is the smoking gun we’ve been looking for,” says Holt, adding that the lack of calcium eventually kills the cell, exacerbating hearing loss.
The study also provided evidence that the two proteins can create channel structures either singly or combined in groups. Holt speculates that different configurations of TMC1 and TMC2 enable different hair cells to be sensitive to different pitch ranges. “There are gradients of expression of these two proteins, which we think are involved in tuning of the sensory cell,” says Holt.
Holt believes that TMC channels may have other physiologic functions in the body, such as blood pressure regulation or sensation of needing to urinate—anything that requires a mechanical sensation as a trigger.
Meanwhile, based on the initial genetic discoveries, a gene therapy study is underway in mice to see whether reintroducing TMC1 and/or TMC2 genes to the inner ear could restore hearing.
Holt’s team is looking for electrical signals in the 8th cranial nerve—indicating that the brain is being signaled—and more importantly, whether the animals respond to sound. Early results are encouraging; Holt asked me to check back in a year.
Related Posts :
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

New research paves the way to a better understanding of telomeres
Much the way the caps on the ends of a shoelace prevent it from fraying, telomeres — regions of repetitive DNA ...
-

New research sheds light on the genetic roots of amblyopia
For decades, amblyopia has been considered a disorder primarily caused by abnormal visual experiences early in life. But new research ...