Beyond appearances: Molecular genetics revises brain tumor classification and care

Scott Pomeroy, MD, PhD, is Neurologist-in-Chief at Boston Children’s Hospital. He practices in the Brain Tumor Center and is a member of the F.M. Kirby Neurobiology Center.
For almost a century, brain tumors have been diagnosed based on their appearance under a microscope and classified by their resemblance to the brain cells from which they are derived. For example, astrocytoma ends with “-oma” to designate that it is a tumor derived from astrocytes. In some cases, especially in children, brain tumors resemble cells in the developing brain and are named for the cells from which they are presumed to arise, such as pineoblastoma for developing cells within the pineal gland or medulloblastoma for developing cells within the cerebellum or brainstem.
In June, the World Health Organization (WHO), which sets the worldwide standard, released an updated brain tumor classification scheme that, for the first time, includes molecular and genetic features.
Going forward, pathologists in hospitals all over the world will diagnose brain tumors not only by their appearance under a microscope, but based on their molecular and genetic signatures. These signatures in some cases establish the underlying mechanisms driving the tumor, and more accurately predict whether the tumor will respond to therapy.
This historic change in brain tumor classification is the result of years of research in labs around the world, including our own.
Not all brain tumors are made the same
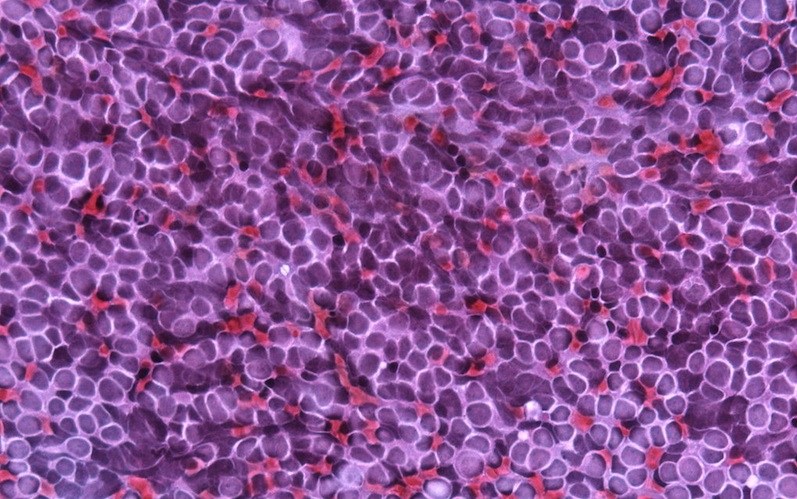
When I began taking care of children with brain tumors at Boston Children’s Hospital in 1991, I quickly realized that some tumors, especially medulloblastomas, had quite varied behavior despite looking very similar under the microscope. About half of the children responded to treatment and are still alive today. But the others progressed and succumbed after variable periods of time, despite the fact that they received identical treatment.
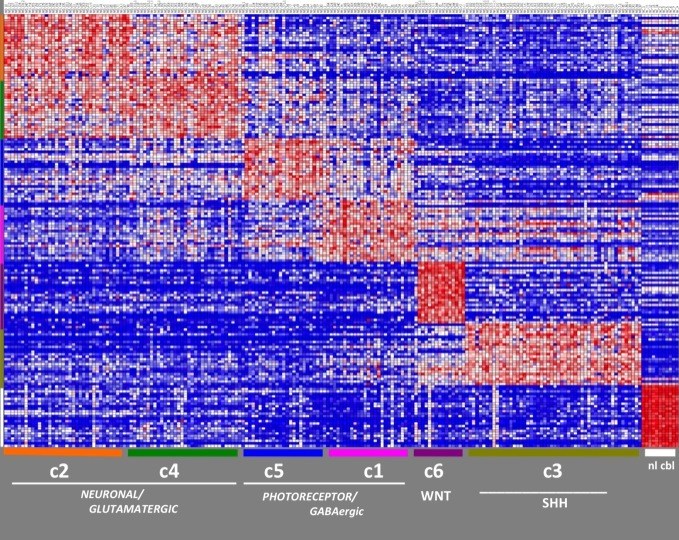
The first clue to this mystery came when my lab discovered in the 1990s that the presence of certain molecules that normally appear during development predict response to medulloblastoma therapy better than the tumor’s appearance under the microscope. In 2002, my lab and colleagues at MIT applied genomics to show that medulloblastomas are actually comprised of subgroups with different molecular profiles and clinical outcomes. The molecular signatures we had found earlier were a manifestation of these subgroups.
Further work by my lab and by colleagues in Toronto, Heidelberg and Memphis further delineated the subgroups. In 2010, we met in Boston, and our consensus — that there are four primary subgroups of medulloblastoma — provided the scientific evidence for the new WHO classification.
Similar schemes are emerging for other types of brain tumors. For example, work that my laboratory did in collaboration with Charles Roberts, MD, PhD, then at Dana Farber Cancer Institute, established that atypical teratoid/rhabdoid tumors (arising in the brain and spinal cord) and other rhabdoid tumors (arising in the kidneys and other sites) all have the same genetic defect.
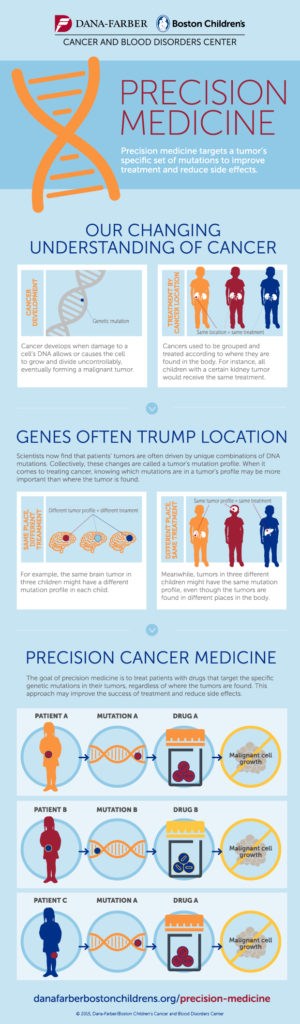
Collectively, these findings reveal that the molecular features of tumors drive their biological behavior, establishing a basis for precision cancer medicine (see infographic). Using the new WHO diagnostic classification, children with brain tumors all over the world can now receive less radiation and chemotherapy if they have a good molecular prognosis, reducing long-term complications, and intensified treatments if they have a molecular subtype known to not respond to conventional therapy.
The genomic approach is also uncovering molecules that drive tumor growth. These can now be specifically targeted with the goal of further reducing radiation and chemotherapy. In the future, we hope that children will not only survive their cancer, but also have the highest quality of life and grow into successful adults.
Read more about precision cancer medicine, and learn more about the Brain Tumor Center at Boston Children’s Hospital.
Related Posts :
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

Forecasting the future for childhood cancer survivors
Children are much more likely to survive cancer today than 50 years ago. Unfortunately, as adults, many of them develop cardiovascular ...
-

Pediatric high-grade gliomas: Research reveals effective targeting with avapritinib
Pediatric high-grade gliomas, particularly H3K27M diffuse midline gliomas (DMG), are aggressive malignant brain tumors with a poor prognosis. ...
-

Mapping cells to create targeted treatments for interstitial lung disease
John Kennedy, MD, MSc, remembers the relative simplicity of his first genetic mapping project. In a Harvard Medical School&...