“Teenage” red blood cells could hold the key to a malaria vaccine
Malaria parasite infection, which affects our red blood cells, can be fatal. Currently, there are about 200 million malaria infections in the world each year and more than 400,000 people, mostly children, die of malaria each year.
Now, studying blood samples from patients treated for malaria at a clinical field station in Brazil’s Amazon jungle, a team of Brazilian and American researchers has made a surprising discovery that could open the door to a new vaccine.
“I noticed that white blood cells called killer T cells were activated in response to malaria parasite infection of immature red blood cells,” says Caroline Junqueira, PhD, a visiting scientist at Boston Children’s Hospital and Harvard Medical School (HMS).
For red blood cells, this activity is unusual.
“Infected red blood cells aren’t recognized by our immune system’s T cells in the same way that most other infected cells of the human body are,” says Judy Lieberman, MD, PhD, chair in the Program in Cellular and Molecular Medicine at Boston Children’s Hospital.
Digging deeper, Junqueira, Lieberman and collaborators have found a completely unexpected immune response to malaria parasites that infect immature blood cells called reticulocytes. The revelation could help to design a new vaccine that might be capable of preventing malaria.
Their findings, published today in Nature Medicine, uncover special cellular mechanisms and properties specific to “teenaged” reticulocytes and a strain of malaria called Plasmodium vivax that enable our T cells to recognize and destroy both the infected reticulocytes and the parasites inside them.
Out of the Amazon
Until now, P. vivax, which is endemic to Brazil, has been especially challenging to study because this particular strain of malaria can’t survive more than 12 hours in lab culture. To do this work, Junqueira needed to make 16 trips into the heart of the Amazon to collect patient blood samples.
P. vivax, like all malarial parasites, is transmitted to humans by mosquitoes. Once inside a human host, the parasites proliferate in the liver before disseminating into the blood stream. There, the parasites infect red blood cells, eventually causing the infected cells to burst. This releases the infectious parasites back into the blood stream where they can infect even more red blood cells. Meanwhile, symptoms escalate: first a cold stage of shivering, then a hot stage of fever and finally a third stage of sweating and fatigue.
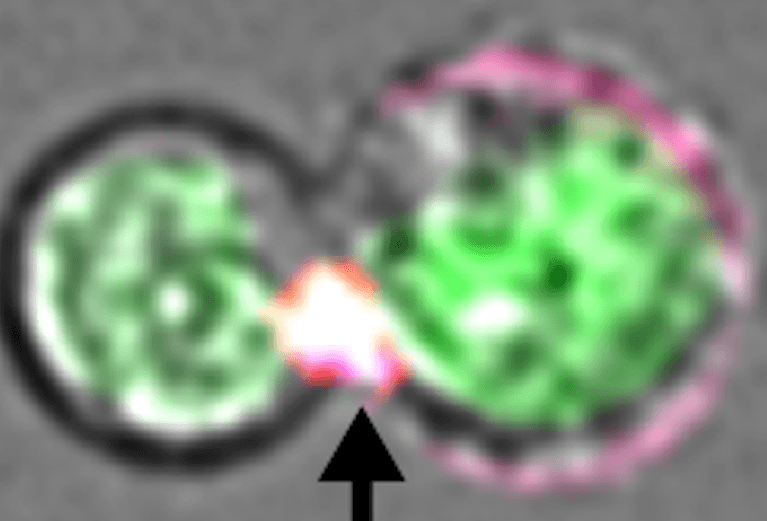
When infected, most of our body’s cells are able to flag down the help of killer T cells by presenting the invading pathogen’s antigens on their cell surface. But mature red blood cells lack the internal cellular machinery to do so. On their path of differentiation from blood stem cells in our bone marrow, red blood cells shed much of their cellular machinery to make room for their primary function of ferrying hemoglobin, an oxygen-carrying molecule, throughout the body.
But reticulocytes aren’t quite full-blown red blood cells yet. Therefore, they still retain some cellular machinery that enables them to present peptide antigens from P. vivax to our immune system’s T cells. The researchers found that this allows T cells to launch an attack on the infected reticulocytes and the malarial parasites within them.
“So far, vaccines to protect against this strain of malaria have been designed to induce only antibodies, but antibody-based vaccines haven’t worked,” says Lieberman, co-senior author on the study. “Until now, other immune responses mediated by T cells weren’t thought to have any protective role against blood-stage malaria infection.”
Something else special
During most infections, our T cells typically use two pore-forming proteins called perforin and granulysin to breach the membranes of our infected cells and the parasites inside them. First, perforin creates pores in our infected cells through which granulysin and death-inducing enzymes pass through. Once inside an infected cell, granulysin bores into the cell membrane of the parasite within, creating an opening that allows the lethal enzymes to induce their fatal effect.
Yet interestingly, Lieberman’s team found that T cells are able to wipe out infected reticulocytes and the P. vivax inside them without using perforin at all.
“This immune response is special,” says Lieberman, who is also a professor of pediatrics at HMS. Lieberman is a “cell death” expert. She has pioneered our understanding of programmed cell death, or apoptosis, which plays an important role in normal growth and development. Apoptosis, for example, is the process by which the cells between our fingers die during development. Without apoptosis, we would all have webbed hands. In her most recent apoptosis research, Lieberman discovered the importance of a protein called PTPN1, which is released from mitochondria during apoptosis. PTPN1 critically promotes RNA decay and cell death. Her findings were published in the June 28 issue of Cell.
The researchers discovered that cholesterol — or lack of it — has a pivotal role in the T cells’ unexpected ability to kill malaria-infected reticulocytes with granulysin alone.
“Granulysin typically only works on bacteria or parasites because it targets cells that don’t contain cholesterol,” says Junqueira, who is a researcher in Lieberman’s lab. In contrast, perforin targets our cells’ membranes, which usually contain abundant amounts of cholesterol.
“But in the case of P. vivax, the parasites actually steal cholesterol away from the reticulocytes they infect. Drained of cholesterol, infected reticulocytes become susceptible to granulysin’s pore-forming mechanisms and perforin isn’t needed to deliver lethal enzymes inside the infected cell.”
Now, looking ahead, Lieberman and Junqueira say that there is translational work to be done.
“This all started in Brazil with Caroline’s clinical observations,” Lieberman says. “Back in the lab, though, we’ve been able to discover new insights into the molecular underpinnings of the immune response to P. vivax. Now, we are collaborating with peptide experts to learn exactly which malaria proteins are recognized by T cells. Our ultimate goal is to design a vaccine that induces both antibody and T cell responses to the parasite.”
Ricardo Gazzinelli, a Brazilian immunologist and co-senior author on the study with laboratories in both Brazil and at UMass Medical Center, was also a key collaborator on this research. He has previously tried to develop a vaccine to protect against P. vivax.
Related Posts :
-

“Observe. Be open.”: How Boston Children’s nurses are changing the future of global health
Ashley Birch, MSN, CPNP, a Boston Children’s pediatric nurse practitioner and Global Nursing fellow, didn’t expect a trash ...
-

Tough cookie: Steroid therapy helps Alessandra thrive with Diamond-Blackfan anemia
Two-year-old Alessandra is many things. She’s sweet, happy, curious, and, according to her parents, Ralph and Irma, a budding ...
-

A new druggable cancer target: RNA-binding proteins on the cell surface
In 2021, research led by Ryan Flynn, MD, PhD, and his mentor, Nobel laureate Carolyn Bertozzi, PhD, opened a new chapter ...
-

Unique data revealed just when Mickey’s heart doctors could operate
When Mikolaj “Mickey” Karski’s family traveled from Poland to Boston to get him heart care, they weren’t thinking ...





