Small samples, big data: A systems-biology look at a newborn’s first week of life

The first week of a baby’s life is a time of rapid biological change. The newborn must adapt to living outside the womb, suddenly exposed to new bacteria and viruses. Yet scientists know surprisingly little about these early changes.
Reporting in Nature Communications, an international research team provides the most detailed accounting to date of the molecular changes that occur during a newborn’s first seven days. The team pioneered a technique to extract volumes of data from a tiny amount of newborn blood — including what genes are turned on, what proteins the body is making and what metabolites are changing.

The work provides a baseline for understanding newborn health and, in particular, the impacts of vaccines on newborns.
“Most infections in the world occur early in life, and newborns have the greatest susceptibility and the worst outcomes,” says Ofer Levy, MD, PhD, director of the Precision Vaccines Program at Boston Children’s Hospital and a senior author on the paper. “This work provides a valuable window into health and disease in the first week of life.”
Tiny sample, big knowledge

The research team includes members of the Expanded Program on Immunization Consortium (EPIC), an international collaboration to employ a systems biology approach to understanding vaccine action in early life. Levy partnered with Beate Kampmann, MD, PhD, of the London School of Hygiene & Tropical Medicine (LSHTM) and the Medical Research Council (MRC)-The Gambia, and Tobias Kollmann, MD, PhD, University of British Columbia (UBC), to establish the consortium. The consortium has its administrative hub in the Precision Vaccines Program.
Previous efforts to gather data on newborn development have been limited by the very tiny blood samples that can safely be taken from newborns. Getting around this challenge required the team to develop new techniques. They partnered with Robert Hancock, PhDof UBC, who led an RNA analysis, and Hanno Steen, PhD of Boston Children’s, who led a proteomics analysis.
Using sophisticated software and less than half a teaspoon of blood, the UBC labs of Hancock and Scott Tebbutt, PhD found thousands of changes over the first week. They saw changes in gene expression and components of immune defense such as interferons, neutrophil function and complement pathways.
“We demonstrated that it’s possible to recruit newborns in a resource-poor setting, obtain small amounts of their blood, process it, ship it, conduct systems biology assays and integrate the results — turning big data into knowledge,” says Levy. “Key to our analysis was comparing each newborn at day 1, day 3 and day 7 to their own baseline condition on the day of birth. That’s when we discovered dramatic molecular changes driven by development.”
An intercontinental effort

The researchers piloted their systems biology approach with a group of infants from The Gambia in West Africa, obtaining permission from village elders and informed consent from mothers in local languages such as Mandinka, Fula and Wolof. They then validated their approach with a group of Australasian newborns. These babies were recruited by William Pomat, PhD, of the Papua New Guinea Institute of Medical Research, and Anita van den Biggelaar, PhD, of the University of Western Australia and the Telethon Kids Institute.
Both cohorts showed a common, highly dynamic developmental trajectory. The changes didn’t appear to occur at random, but instead seemed to follow an age-specific pathway.
“This common trajectory is exciting as it allows us to ask bigger questions about the differences between different populations and the impact of interventions such as vaccines on development,” says Levy.
Optimizing vaccines in early life
The study establishes a baseline for health and disease in early life that can help investigators measure responses to medical interventions and the effects of factors like diet, disease and maternal health.
Levy especially wants to study the impact of immunization. Newborns’ responses to immunization are distinct from those of older individuals. Much of Levy’s work focuses on optimizing the use and benefit of vaccines in early life.
“Currently, most vaccines are developed by trial and error,” says Levy. “We seek deep molecular insight into vaccine function in early life so we can better develop infant vaccines for the future.”
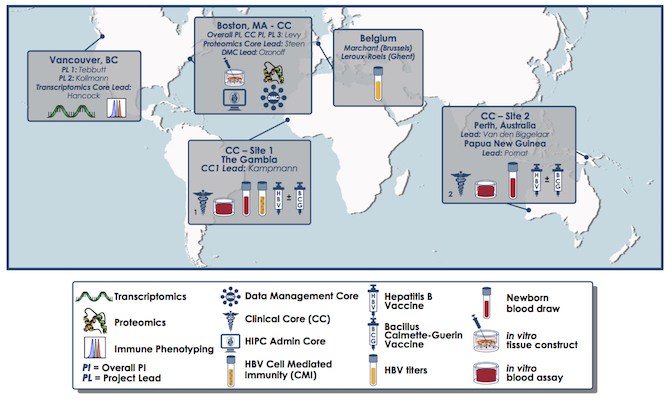
The study was supported in part by the NIH’s National Institute of Allergy and Infectious Diseases as part of the Human Immunology Project Consortium, and by the Precision Vaccines Program. Co-first authors were Amy Lee, Casey Shannon and Nelly Amenyogbe of UBC; Tue Bennike of Aalborg University, Denmark; Joann Diray-Arce of Boston Children’s and Olubukola (“Bukky”) Idoko of LSHTM/MRC-The Gambia. Co-senior authors were van den Biggelaar, Steen, Tebbutt, Kampmann, Levy and Kollmann.
More on the Precision Vaccines Program
Related Posts :
-

The ‘Trach Chapter’: Isabella’s journey with bronchopulmonary dysplasia
Isabella’s life has been anything but ordinary. Born at just 27 weeks gestation and weighing only 1 pound, 4 ounces, Isabella has ...
-

Study highlights the severity of acute necrotizing encephalopathy in kids with the flu
For most children, influenza (flu) usually means unpleasant symptoms like a fever, sore throat, and achy muscles. But for a ...
-

New genetic insights could change how we treat, and talk about, polycystic ovary syndrome
Polycystic ovary syndrome (PCOS) has long been viewed as a hormonal disorder affecting women of reproductive age. However, ongoing research ...
-

Modifying macrophages in the lung could head off pulmonary hypertension
In the 1980s, when Stella Kourembanas, MD, began her career in neonatology, she cared for newborns with pulmonary hypertension, a ...