Earlier treatment may help reverse autism-like behavior in tuberous sclerosis

New research on autism has found, in a mouse model, that drug treatment at a young age can reverse social impairments. But the same intervention was not effective at an older age.
The study is the first to shed light on the crucial timing of therapy to improve social impairments in a condition associated with autism spectrum disorder. The paper, from Boston Children’s Hospital, the University of Texas, Harvard Medical School and Toronto’s Hospital for Sick Children, was published today in Cell Reports.
Tuberous sclerosis and autism
Many of the hundreds of genes that likely regulate complex cognitive and neuropsychiatric behaviors in people with autism still remain a mystery. However, genetic disorders such as tuberous sclerosis complex, or TSC, are providing clues. Patients often have mutations in the TSC1 or TSC2 gene, and about half develop autism spectrum disorder.
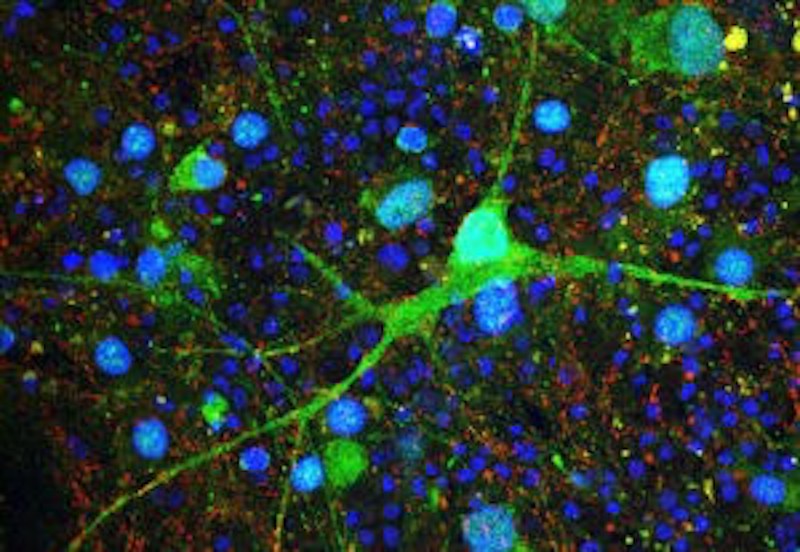
The investigators, led by Peter Tsai, MD, PhD, at UT Southwestern Medical Center, used a mouse model in which the TSC1 gene is deleted in a region of the brain called the cerebellum.
“There were several mouse models of TSC previously published, but they all had seizures and died early in life, making it difficult to study social cognition,” says Mustafa Sahin, MD, PhD, who directs the Translational Neuroscience Center and the Translational Research Program at Boston Children’s and was the study’s senior investigator. “That is one reason why we turned to knocking out the TSC1 gene only in cerebellar Purkinje cells, which have been implicated in autism. These mice have normal lifespans and do not develop seizures.”
Timing is everything
The new research fed off a previous study published in 2012. In that study, Sahin and colleagues treated the mutant mice starting in the first week of life with rapamycin, a drug approved by the FDA for brain tumors, kidney tumors and refractory epilepsy associated with TSC. They found that they could rescue both social deficits and repetitive behaviors.
But when a similar drug, everolimus, was tested in children with TSC, neurocognitive functioning and behavior didn’t significantly improve. Sahin and his colleagues wondered whether there was a specific developmental period during which treatment would be effective.
The new mouse study delineates not only the timeframe for effective rapamycin treatment of certain autism-relevant behaviors, but also some of the cellular, electrophysiological and anatomic mechanisms for these sensitive periods.
“We found that treatment initiated in young adulthood, at 6 weeks, rescued social behaviors, but not repetitive behaviors or cognitive inflexibility,” says Sahin.
More importantly, neither the social deficits nor the repetitive behaviors responded when the treatment was started at 10 weeks.
Using advanced imaging, the researchers went on to show that the rescue of social behaviors correlates with reversal of specific MRI-based structural changes, cellular pathology and Purkinje cell excitability. Meanwhile, motor learning rescue appeared independent of Purkinje cell survival or rescue of cellular excitability.
A new clinical trial?
Based on the mouse findings, Sahin is now seeking funds to test whether early treatment can improve a broad range of autistic-like behaviors in children with TSC. Specifically, he’ll explore whether treatment as early as 12 to 24 months can help prevent both social deficits and repetitive inflexible behaviors. He hopes to see better results than in the earlier clinical trial, which involved children ages 6 to 21.Treatment initiated at 6 weeks rescued social behaviors, but not repetitive behaviors or cognitive inflexibility.
Past research indicates that different autism-related disorders may have different windows of treatment. For example, animal studies of Rett syndrome suggest that treatment can be effective relatively late in life and still improve neurological outcome.
Peter Tsai, first author on the current paper, is a former postdoctoral fellow in the Sahin Lab at Boston Children’s. Other key contributors include Wade Regehr, HMS professor of neurobiology, and Jason Lerch, associate professor at the University of Toronto and research scientist at SickKids. The study was supported by the Nancy Lurie Marks Foundation, the Hearst Foundation, the National Institute of Neurologic Disorders and Stroke, the Tuberous Sclerosis Alliance, the Boston Children’s Hospital Translational Research Program and Intellectual and Developmental Disabilities Research Center, the Canadian Institute for Health Research, Ontario Brain Institute and Brain Canada.
Related Posts :
-

The hidden burden of solitude: How social withdrawal influences the adolescent brain
Adolescence is a period of social reorientation: a shift from a world centered on parents and family to one shaped ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...
-

The thalamus: A potential therapeutic target for neurodevelopmental disorders
Years ago, as a neurology resident, Chinfei Chen, MD, PhD, cared for a 20-year-old woman who had experienced a very ...
-

Could peripheral neuropathy be stopped before it starts?
An increase in high-fat, high-fructose foods in people’s diets has contributed to a dramatic increase in type 2 diabetes. This, ...