Childhood brain cancer: Learning to divide and conquer
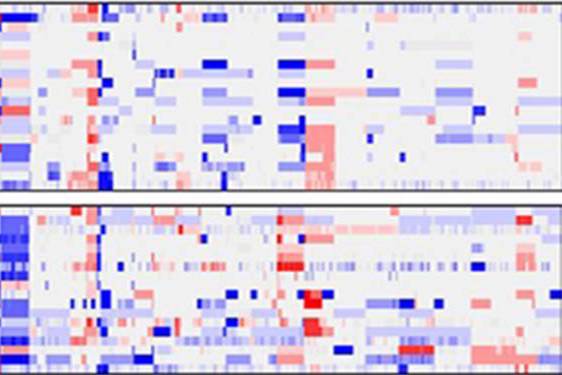
Diversity is good in populations of people, but when it comes to cancer, it’s bad news. In the case of medulloblastoma—the most common malignant brain cancer in children—tumor diversity has been one of the greatest barriers to designing effective treatments.
Now, in the largest genomic study of human medulloblastomas ever, Children’s researchers and their collaborators have subdivided the cancer into six different diseases—each with distinct molecular “fingerprints.” Knowledge of these tumor subtypes will improve neurologists’ ability to direct and individualize treatment. One subtype, carrying the worst prognosis, had never before been characterized.
The study, led by Children’s neurologist Yoon-Jae Cho, MD, and Neurologist-in-Chief Scott Pomeroy, MD, PhD, is expected to bring the first biomarkers for medulloblastoma to the clinic. The international Children’s Oncology Group already plans to apply some of the new data to subtype patients in upcoming clinical trials.
When more is better
Medulloblastomas originate in the cerebellum, a part of the brain that controls motor coordination and other essential functions, and have an overall 40 to 50 mortality rate. The current standard of care after surgical removal is a blend of radiation and chemotherapy—treatments that pose the potential for long term neurological and cognitive side effects.
With biomarkers indicating a tumor subtype and its known survival rate, doctors and families could weigh the risks of specific treatments with the benefits. Patients needing the most aggressive interventions could get them, while patients with better prognoses could try lower risk interventions.
Research aimed at personalization of cancer treatment requires a large number of tumor samples. In this case, 194 samples in total were gathered through collaboration with many institutions. “Over the past two decades, Scott has worked hard to accrue samples here at Children’s Hospital and has also created national tumor banks,” Cho explains. “This gives us much better resolution in determining the number of molecular subtypes represented in medulloblastoma.”
A three-way look at the genome
Earlier studies by Pomeroy and others showed that different gene activity patterns in tumor samples correlate with different outcomes (basically, a better or worse prognosis). The new findings, published online November 22 in the Journal of Clinical Oncology, go further by showing differences not only in gene activity, but in the actual genetic makeup of the different subtypes. For example, the subtype with the worst prognosis is unique in having extra copies of MYC, a well-known oncogene. The team also found distinct differences in the activity of microRNAs, regulatory molecules that usually dampen the activity of their target genes, across the different medulloblastoma subtypes.
“Until recently, all we could do is look at brain tumors under the microscope and say what they look like,” says Pomeroy. “Now we actually have precise molecular signatures and we know what subtype of medulloblastoma it is. And we can say something about what the prognosis might be.”
Related Posts :
-

‘Challenge accepted’: Sophia takes on a brain tumor
In 2023, Sophia Mordini landed the role of a lifetime. A competitive dancer, the 12-year-old would play Clara in her company’...
-

A true hero’s journey: How a team approach helped Wolfie overcome pancreatitis
Wolfgang, affectionately known as “Wolfie,” is a bright and energetic 7-year-old with a quick wit and a love for making ...
-

A case for Kennedy — and for rapid genomic testing in every NICU
Kennedy was born in August 2025 after what her parents, John and Diana, describe as an uneventful pregnancy. Soon after delivery, ...
-

The journey to a treatment for hereditary spastic paraplegia
In 2016, Darius Ebrahimi-Fakhari, MD, PhD, then a neurology fellow at Boston Children’s Hospital, met two little girls with spasticity ...





